Costs involved in compliance with new endoscope reprocessing guidelines
Article information
Abstract
Background/Aims
In March 2022, the Association for the Advancement of Medical Instrumentation (AAMI) released the American National Standards Institute (ANSI)/AAMI ST91:2021, their latest update on comprehensive, flexible, and semirigid endoscope reprocessing. These updated standards recommend the sterilization of high-risk endoscopes when possible and provide new recommendations for the precleaning, leak testing, manual cleaning, visual inspection, automated reprocessing, drying, storage, and transport of endoscopes.
Methods
ANSI/AAMI ST91:2021 was compared with ANSI/AAMI ST91:2015 for major reprocessing differences that result in either time and/or cost increases. Time estimates were captured by explicit recommendation inclusion or taken from the literature. All the costs were estimated using publicly available resources.
Results
The updated standards represent a potential 24.3-minute and 52.35 to 67.57 United States dollars increase per procedure in terms of reprocessing time and spending, respectively, not including capital investments. Capital costs per procedure were highly dependent on the procedure volume of the facility.
Conclusions
The new AAMI standards recommend several major changes, such as sterilization, for facilities to reprocess and manage endoscopes between uses. As more facilities increase their reprocessing methods to reflect the updated standards, they do so at a cost and introduce several delays. As the reprocessing landscape evolves, facilities should consider their true costs and alternative solutions, such as single-use endoscopes.
INTRODUCTION
Endoscopies involve the direct insertion of a visualization device into an organ or body cavity, and have been performed since the 1850s.1 Endoscopic examinations are presently performed in a variety of healthcare settings and facilities, and are commonly utilized for a multitude of indications, including procedures that examine the lung (bronchoscopy), colon (colonoscopy), bladder and urethra (cystoscopy and ureteroscopy), upper gastrointestinal tract (esophagogastroduodenoscopy), and upper airway (rhinolaryngoscopy). Most endoscopes used in these procedures are reusable, and thus require extensive cleaning, or reprocessing, between uses. Despite best efforts, endoscope-related cross-contamination and infection continue to be serious threats to patient safety, as they cannot be completely sterilized again after their first use. Between 2019 and 2021, reusable endoscopes showed a 125% increase in Manufacturer and User Facility Device Experience (MAUDE) reports related to infection control issues.2 Additionally, peer-reviewed literature has shown readmission and infection rates related to reusable endoscopes to be 7.7% and 2.8%, respectively.3,4
To help curb endoscope cross-contamination and infections, regulatory bodies and advocacy organizations such as the Food and Drug Administration (FDA), Association of Perioperative Registered Nurses (AORN), Association for Professionals in Infection Control and Epidemiology, and Association for the Advancement of Medical Instrumentation (AAMI) have released new standards and guidelines for endoscope reprocessing. While these new recommendations are intended to remedy issues, they are often met with slow adoption and/or noncompliance. For example, a 2019 study showed that over 65% of institutions were noncompliant with previous reprocessing guidelines.5 Additional time, costs, and staff shortages are some of the most common underlying causes of slow adoption and noncompliance.6 To help combat this, the FDA has recommended that healthcare facilities start to transition from fully reusable endoscopes to disposable options, and when not possible, shift towards more robust forms of reprocessing, such as sterilization by ethylene oxide (EtO), liquid chemicals, or gas plasma.7 This shift is especially crucial because there have been multiple recalls of endoscopes, such as the one by a large endoscope manufacturer in April 2022, as a result of reprocessing failures following high-level disinfection (HLD), resulting in inadequate reprocessing of urological endoscopes and potentially increasing the risk of patient infection.8 Furthermore, the additional steps required to sufficiently sterilize endoscope can increase costs by up to 216 United States dollars (USD) per procedure.9
In March 2022, AAMI released the American National Standards Institute (ANSI)/AAMI ST91:2021, their latest update on comprehensive flexible and semirigid endoscope processing, which was the result of inputs from multiple stakeholders from AAMI, AORN, researchers, clinicians, and industry professionals.10 AAMI has served as a critical thought leader in the medical device space for decades and is the primary source of consensus standards in this industry. ANSI/AAMI ST91:2021 was their first update in 7 years and contains several important new recommendations for healthcare facilities to adopt and standards to comply with. The updated standards provide new recommendations for the precleaning, leak testing, manual cleaning, automated reprocessing, drying, storage, and transport of a wide array of endoscopes. Additionally, these standards recommend the sterilization of high-risk endoscopes whenever possible. AAMI defines high-risk endoscopes as “endoscopes that have been associated with infectious outbreaks including those that are difficult to process and increase the risk of incomplete clearance of contaminating infectious organisms, including bronchoscopes, cystoscopes, duodenoscopes, endobronchial ultrasound endoscopes, linear ultrasound endoscopes, ureteroscopes, and others as determined by the facility.”9 While not enforceable, the recommendations serve to direct healthcare professionals and often form the basis for the protocols that accredited facilities must follow.
Prior to the release of ANSI/AAMI ST91:2021, reprocessing a reusable endoscope via a single HLD (sHLD) with an automated endoscope reprocessor cost roughly 80 USD, not including capital costs.9 As more facilities worldwide adopt the new recommendations, the cost and time requirements will dramatically increase due to the >20 additional steps outlined for compliance. Although the new standards recommend sterilizing endoscopes when possible, this is not always a viable solution. Healthcare facilities need to enhance their reprocessing methods to meet the standards set for patient safety, regardless of additional cost and/or time. The purpose of the following evaluation is to better understand how the new recommendations will impact facilities, given the time needed to clean and reprocess reusable endoscopes, as well as the associated costs.
METHODS
Major changes in the reprocessing recommendations were identified between ANSI/AAMI ST91:2021 and ANSI/AAMI ST91:2015. AAMI was utilized for this analysis given its aforementioned reputation in the medical device industry. To be included in the comparison, the newly recommended changes must add either cost or time to the reprocessing cycle of reusable endoscopes. Time estimates were captured by explicit inclusion in the recommendations or obtained from the literature. When possible, the costs (USD) were estimated using publicly available literature. A literature search was performed to establish the average and range of potential values for certain costs, such as monitoring water supply quality and new reprocessing sinks. To calculate time costs, labor costs were obtained from the United States Bureau of Labor Statistics. For recommendations that did not require hands-on activities, such as extended drying times, no labor costs were incurred. Additional literature was reviewed to determine the costs that may be sustained due to sterilization.
Ethical statements
Not applicable for studies not involving humans or animals.
RESULTS
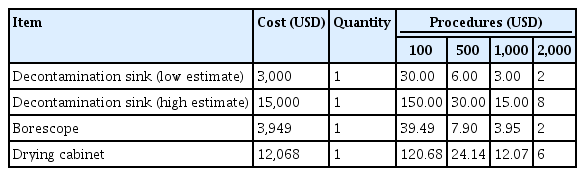
This examination only sought to estimate the incremental time and costs of upgrading a facility’s reprocessing methods and standards to the most recent AAMI standards on a per-cycle basis. No steps were found to reduce either the per-cycle cost or time required to perform endoscopic reprocessing. All incremental per-cycle capital costs, such as new reprocessing sinks, drying cabinets, and borescopes required to update the facility, were dependent on other variables, mainly procedure volume, for calculation.
The updates in the new standards represent a potential 24.3-minute increase in reprocessing. This encompassed time increases for environmental monitoring (5.4 minutes), leak testing (0.5 minutes), endoscope-cleaning verification (8.4 minutes), and drying (10 minutes). To calculate the appropriate cost associated with the extra time, an hourly rate of 20.40 USD was used.11
The updates in the new standards also represent a potential 52.35 to 67.57 USD increase in per-procedure reprocessing spending, not including capital investments. This is a sum of the environmental (11.88 USD), transport (0.98 USD), leak-testing (0.17 USD), cross-contamination and infection-control (0.38–15.60 USD), drying (38.51 USD), and storage (0.43 USD) costs (Table 1). The capital investments can be as high as 15,000 USD for a new reprocessing sink, 4,000 USD for a borescope, and 12,000 USD for a drying cabinet (Table 2).12
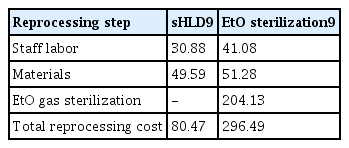
Additionally, the guidelines recommend sterilizing high-risk endoscopes when possible, and as a result, may incur an even greater cost. A 2021 study by Bomman et al.9 examined the costs of both sHLD and EtO sterilization at two medical institutions in the US. The study found that performing sHLD costs 80.47 USD per cycle while EtO sterilization can cost 296.49 USD per cycle.9 By transitioning from sHLD to EtO sterilization, facilities may increase their reprocessing costs by 216.49 USD and add approximately 46 minutes of staff labor time per cycle (Table 3).9
DISCUSSION
If a facility adopts all additional reprocessing steps to reflect the new AAMI standards, it can expect to incur an additional cost of 52.35 to 67.57 USD and a delay of 24.3 minutes per cycle, not including capital investments. Despite this increase, it is important to note that the guidelines recommended sterilizing high-risk endoscopes when possible, thus increasing the reprocessing costs by 216.49 USD, adding approximately 46 minutes of staff labor time per cycle.9,10 Despite this increase in cost, the authors felt that the costs listed may be conservative, given that their analysis did not include the required capital, service, and necessary repair costs. Additionally, the study noted that the additional time required for EtO sterilization did not include the time required to run the sterilizer, which can be 15 to 16 hours.9
Two major limiting factors in the implementation of sterilization are cost and availability. For instance, only 20% of hospitals in the US have the ability to perform EtO sterilization on-site.9 Instead of constructing a new sterilization infrastructure onsite at a high cost, facilities can opt for a third-party vendor to undertake the process. This process usually entails shipping endoscopes after use to a sterilization company via a carrier service. In addition to adding extra steps to the reprocessing, shipping adds additional costs and time to the reprocessing cycle.9 This method also adds to the extra risk of damage due to frequent shipping, increasing repair costs, and thus the need to purchase extra endoscopes in case some become unusable.9 While a facility can perform sterilization in-house, it may still be subject to paying for repairs in as little as every eight reprocessing cycles due to glue blistering and every 23 cycles due to insertion tube cracking.13 Even without the risk of extra repair, the added downtime of enhanced reprocessing techniques may require facilities to increase their endoscope supply 3.4-fold to maintain their current procedural volume.9
If sterilization is not implemented, the costs of upgrading to new AAMI standards can cost between 52.35 to 67.57 USD and add roughly 24 minutes to the reprocessing process, on top of the 76 minutes and 80.47 USD facilities already face.12 In addition to the aforementioned per-procedure costs, they must consider the additional capital equipment required to meet the new recommendations. Furthermore, the per-procedure costs of these purchases are likely to be higher at lower-volume facilities, given that the costs are distributed over fewer procedures. Table 4 outlines how these costs differ for procedure volumes of 100, 500, 1,000, and 2,000.
Although some facilities will see smaller per procedure capital cost increases due to procedure volume, all facilities, regardless of size, will incur the additional reprocessing costs and delays mentioned above. Table 5 highlights the ranges of facilities with 100, 500, 1,000, and 2,000 procedures could see, considering all costs.
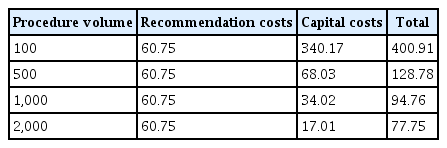
Reprocessing and capital costs per procedure at multiple procedure volumes (unit: United States dollar)
While considering the aforementioned updated steps, an important factor to consider is noncompliance or breaches in reprocessing protocols. If a healthcare facility upgrades its reprocessing methods to become more robust, many do nothing to ensure that all steps are performed correctly. Lack of reprocessing compliance is a serious issue in the endoscopy space, and a Joint Commission review found 65.58% of hospitals to be noncompliant in reducing the risk of infections associated with medical equipment, devices, and supplies because of improper sterilization or HLD.5 An example of this can be found in a recent (2022) case-study examining a urology practice, common reasons where common reasons for noncompliance were the absence of precleaning following a procedure, improper documentation of materials and time periods of steps, omission of proper disinfectant flushing, storing without an alcohol rinse, and lack of training documentation by reprocessing staff.14 While issues such as these do not occur at every healthcare facility, increasing the number of steps required to fully reprocess an endoscope will likely increase the chances of noncompliance at more facilities around the country and, in turn, increase the chances of patient safety being compromised.
Lastly, a literature review conducted in 2020 found that the contamination rates for duodenoscopes after sHLD and EtO sterilization were 15.25% and 9.20%, respectively.15 While this study shows that EtO sterilization is superior to sHLD in reducing contamination risk, neither method can eliminate the risk of cross-contamination and infections. One of the reasons for the inability to eliminate cross-contamination between endoscopes is the ubiquitous presence of damage and debris.16 An examination in 2022 revealed that 100% of the endoscopes examined had some form of visible damage and debris.16 The most common types of damage and debris were scratches (88%), channel peeling (80%), band disintegration (80%), retained fluids (52%), and dents (40%).16 Additionally, the study found that because of this damage and debris, the effectiveness of reprocessing could be compromised.16 While the results of this study create a cause for concern, especially for the health and safety of patients, they also reiterate the need for additional borescoping steps mentioned in the new AAMI standards. This was particularly evident in a March 2022 MAUDE report outlining several lung transplant patients who were infected with Mycobacterium immunogenum, resulting in the death of one patient.17 By incorporating visual inspection into the normal reprocessing cycle, endoscopes with internal damage and debris, and thus a lower chance of proper reprocessing, are less likely to be reused on patients.
As more steps to existing guidelines, recommendations for sterilization, and options, including single-use devices, come to the market, facilities are given the option to reevaluate their current endoscopy processes. Single-use endoscopes, for example, may provide facilities an alternative to the costly and timely reprocessing steps required to upgrade to new guidelines and maintain current procedural volumes without a large financial investment. These single-use endoscopes forgo the added expenses required to meet standards because they are disposed of after each use and, thus are not reprocessed. The cost of single-use endoscopes can vary significantly depending on the type of endoscope purchased. For example, single-use bronchoscopes can cost 260 USD per unit while single-use duodenoscopes can cost 3,000 USD per unit.18,19 Given their differences in cost structure (capital vs. operational), comparing the costs of reusable and single-use endoscopes at the facility level is highly dependent on many variables, such as procedure volume, repair costs, amount of capital, and reprocessing method. As the single-use market grows, facilities should perform analyses to decide which path is best for them and understand their own costs.
Finally, there were several limitations that must be addressed. First, the cost data for the new steps were obtained from several healthcare institutions and reprocessing vendors. Depending on the current purchasing agreements contracted by a facility, costs may not be generalizable to all facilities. Another limitation is the omission of necessary training and education costs to ensure that all staff members perform the new reprocessing steps correctly. In addition, this analysis did not consider the costs of the utilities required to properly undergo these changes during reprocessing. The study also did not consider the potential decreases in endoscope-related cross-contamination or infection costs. As the robustness of reprocessing increases, it is expected that these costs will decrease but will never be eliminated. It is important to note that studies have not yet fully evaluated the long-term impact of the new additions to the guidelines, and it is unclear how these will impact cross-contamination. A cost review of current reprocessing and sterilization techniques should be undertaken to provide insights into their per-procedure costs. To properly observe the impact of the new standards, a full microcosting analysis comparing the statistics before and after adopting the guidelines should be undertaken. This would help limit the influence of generalizations and uncover any process impacts they may have on operations.
The new AAMI reprocessing standards represent a clear goal for improving patient safety via more stringent reprocessing. However, these additional steps incur significant monetary and time costs for the facility, which may result in incremental costs of approximately 60 USD and an additional 24 minutes per reprocessing cycle. Moreover, facilities may be required to buy more capital equipment, such as new endoscopes, sinks, and drying cabinets, to keep up with new standards, offset any delays that may arise, and maintain their current procedural volumes. As more single-use endoscopes enter the market, facilities should consider the impact they may have.
Notes
Conflicts of Interest
Ambu USA is a medical device company which produces single-use devices for hospitals. The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: all authors; Data curation: DH; Formal analysis: DH; Investigation: DH; Methodology: all authors; Project administration: all authors; Resources: CC; Supervision: CC; Validation: CC; Writing–original draft: DH; Writing–review & editing: CC.