Current status and clinical outcome of endoscopic hemostatic powder in gastrointestinal bleeding: a retrospective multicenter study
Article information
Abstract
Background/Aims
Few multicenter studies have investigated the efficacy of hemostatic powders in gastrointestinal (GI) bleeding. We aimed to investigate the clinical outcomes of hemostatic powder therapy and the independent factors affecting rebleeding rates.
Methods
We retrospectively recruited patients who underwent a new hemostatic adhesive powder (UI-EWD; Next-Biomedical) treatment for upper and lower GI bleeding between January 1, 2020 and March 1, 2023. We collected patients’ medical records and bleeding lesions. The primary outcomes were clinical and technical success rates, and the secondary outcomes were early and delayed bleeding rates, refractory bleeding rate, mortality rate, and factors affecting early rebleeding rates.
Results
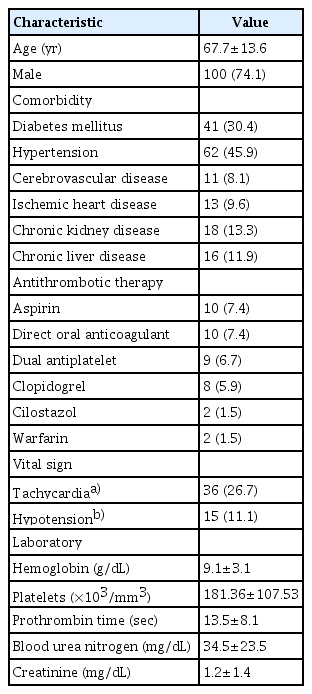
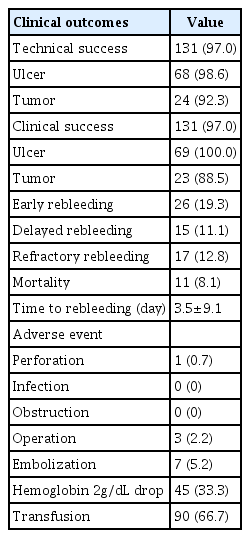
This study enrolled 135 patients (age: 67.7±13.6 years, male: 74.1%) from five hospitals. Indications for UI-EWD were peptic ulcers (51.1%), post-procedure-related bleeding (23.0%), and tumor bleeding (19.3%). The clinical and technical success rates were both 97%. The early, delayed, and refractory rebleeding rates were 19.3%, 11.1%, and 12.8%, respectively. Initially elevated blood urea nitrogen (BUN) levels (p=0.014) and Forrest classification IA or IB compared with IIA or IIB (p=0.036) were factors affecting early rebleeding.
Conclusions
UI-EWD showed high clinical and technical success rates; however, rebleeding after UI-EWD therapy in patients with initially high BUN levels and active bleeding, according to the Forrest classification, should be considered.
INTRODUCTION
Currently, the endoscopic hemostatic powder is used to treat bleeding peptic ulcers, post-procedure-endoscopic mucosal resection- or endoscopic submucosal dissection-related bleeding, hemostasis failure in conventional endoscopic modalities, tumor bleeding, and even bleeding from non-gastrointestinal (GI) tumors.1-18 The effectiveness of hemostatic powder in temporarily controlling massive bleeding from non-GI tumors has been reported.1 Several studies have investigated the efficacy of hemostatic powders.3-16 In 2018, the Asia-Pacific Working Group recommended endoscopic hemostatic powder as a valuable treatment for controlling non-variceal upper GI bleeding with low evidence, especially among inexperienced endoscopists.2 For inexperienced experts, the hemostatic powder could be helpful in that it causes a coagulation process as the hemostatic agent adheres to the lesion surface, and it can relatively easily reach locations (such as large-sized lesions, retroflexed position, angulated digestive segments, or complex postsurgical anatomy) that are difficult for through-the-scope or over-the-scope clips to access.19 According to a recent systematic review and meta-analysis including 20 studies in 2020, the technical and clinical success rates of hemospray (TC-325; Cook Medical) therapy were 97% and 91%, respectively.3
In Korea, a study on the effect of a new hemostatic adhesive powder (UI-EWD; Next-Biomedical) on upper and lower GI bleeding and upper GI tumor bleeding has been published,4-6 and the effectiveness of polysaccharide hemostatic powder in non-variceal upper GI bleeding and in the prevention of bleeding after endoscopic submucosal dissection has been reported.7,8 However, most of these studies are single-institution-based studies, and there are only a few multicenter studies on the efficacy of endoscopic hemostatic powder for GI bleeding. Hence, we aimed to investigate the clinical outcomes of hemostatic powder therapy and the independent factors affecting rebleeding rates in patients treated with hemostatic powder.
METHODS
This retrospective, multicenter study included five hospitals (Kangdong Sacred Heart Hospital, Chonnam National University Hwasun Hospital, Korea University Guro Hospital, Yeouido St. Mary’s Hospital, and Seoul St. Mary’s Hospital) in Korea. We recruited patients treated with endoscopic hemostatic powder (UI-EWD) for upper and lower GI bleeding between January 1, 2020 and March 1, 2023. Most hemostatic powders were used after conventional endoscopic therapy failed, and only 3% of patients were treated with hemostatic powder alone without conventional therapy.
We collected medical record data on patients’ clinical information, baseline characteristics, and bleeding lesions. The patient characteristics included age, sex, underlying diseases, presence or absence of antithrombotic therapy, initial systolic and diastolic blood pressure, heart rate (HR), and laboratory findings. We analyzed patient data, including initial hemoglobin, platelet count, prothrombin time, blood urea nitrogen (BUN), and creatinine levels. Endoscopic bleeding lesions were classified according to the location, cause of bleeding, size of bleeding sites, and the Forrest classification according to the bleeding pattern. Prior hemostatic methods included endoscopic injection therapy with hypertonic saline-epinephrine, thermal treatment (argon plasma coagulation), and endoscopic hemostatic clipping.
Definition
We classified the clinical results into five major categories: technical success, clinical success, early rebleeding, delayed rebleeding, and refractory bleeding. Technical success was defined as the time when the catheter had not been blocked, and the hemostatic agent was successfully applied. Clinical success was defined as immediate hemostatic success following the use of hemostatic agents. Early rebleeding was defined as bleeding occurring within 72 hours at the site where the hemostatic agent was used. Delayed rebleeding was defined as bleeding at the target site for >72 hours after applying the hemostatic powder. Refractory bleeding was defined as continued bleeding despite the use of hemostatic tools.3
Outcomes
The primary outcomes were clinical and technical success rates. Secondary outcomes were early (<72 hours) and delayed (>72 hours) rebleeding rates, refractory bleeding rate, mortality rate, and independent factors affecting early rebleeding rate. We investigated the time to rebleeding and adverse events after using endoscopic hemostatic powder for bleeding control. Among the adverse events, we checked for perforation, infection, obstruction, cases of operation, embolization, and whether the hemoglobin level decreased by ≥2 g/dL in the laboratory.
Statistical analysis method
Descriptive statistics were used to investigate patients’ basic characteristics, and the results of general descriptive data were described as mean±standard deviation or median and interquartile range. Logistic regression analysis was conducted to identify independent factors predicting treatment failure. All analyses were conducted using IBM SPSS statistical software ver. 19.0 (IBM Corp.), and a p<0.05 was considered statistically significant.
Ethical statements
This study was conducted in accordance with the research plan approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (IRB no. 2022-08-019).
RESULTS
Baseline characteristics of patients
This study enrolled 135 patients from five hospitals. The mean age of the participants was 67.7±13.6 years, and 100 patients (74.1%) were male. Antithrombotic therapy was administered to 42 patients (31.1%). Patients’ mean systolic and diastolic blood pressure was 118.9±21.7 mmHg and 69.9±13.2 mmHg, respectively. Fifteen patients (11.1%) had a systolic blood pressure of <90 mmHg. The mean HR was 88.9±18.4 bpm, and 36 patients (26.7%) had tachycardia (>100 bpm). Details are presented in Table 1.
Baseline characteristics of bleeding lesion
Upper and lower GI bleeding was observed in 124 (91.9%) and 11 (8.1%) patients, respectively. Among the upper GI bleeding cases, the gastric body (37.1%) was the most common location, followed by the antrum (28.2%), duodenum (26.6%), esophagus (6.5%), and fundus and cardia (1.6%). Among the 11 patients with lower GI bleeding, three had bleeding from the sigmoid colon and eight from the rectum.
The mean size of the bleeding lesion was 28.9±19.3 mm, and the most common indication was peptic ulcer bleeding (51.1%), followed by post-endoscopic mucosal resection/endoscopic submucosal dissection/endoscopic sphincterotomy bleeding (23.0%) and tumor bleeding (19.3%). The proportions of initial Forrest classifications were as follows: Forrest IA (15.6%), IB (57.0%), IIA (20.7%), and IIB (6.7%).
The treatment modality before hemostatic powder was as follows: epinephrine injection with hemoclipping; 38 (28.1%), epinephrine injection only; 24 (17.8%), hemoclipping only; 22 (16.3%), epinephrine injection with thermal therapy; 19 (14.1%), epinephrine injection with thermal therapy and hemoclipping; 18 (13.3%), thermal therapy only; seven (5.2%), thermal therapy with hemoclipping; two (1.5%), and endoscopic variceal obliteration; one (0.7%). In addition, four (3.0%) patients received initial UI-EWD therapy without pretreatment. The patients with initial UI-EWD therapy had bleeding from sigmoid colon cancer in one patient, gastric cancer in two patients, and postoperative bleeding after pylorus-preserving pancreaticoduodenectomy in one patient. Most of them had bleeding in a wide range rather than a limited range; therefore, UI-EWD was used initially in four patients. Table 2 summarizes the characteristics of the bleeding lesions.
Clinical outcomes of UI-EWD therapy
The clinical and technical success rates were both 97%. Technical and clinical success rates differed between ulcers and tumors. The early and delayed rebleeding rates were 19.3% and 11.1%, respectively. Refractory bleeding and mortality rates were 12.8% and 8.1%, respectively. The mean duration of rebleeding among patients was 3.5±9.1 days. Regarding the adverse events, one patient had a perforation, and none had an infection or obstruction. Three (2.2%) patients underwent surgery, and seven (5.2%) underwent embolization. Hemoglobin levels decreased by 2 g/dL in 45 (33.3%) patients, and 90 (66.7%) patients received blood transfusions after endoscopy. The average follow-up duration after the endoscopic hemostatic procedure was 3.5±3.9 months. The data are summarized in Table 3.
Factors affecting early rebleeding rate in patients using UI-EWD
In the multivariate analysis, the factors affecting early rebleeding rate in patients using UI-EWD were initially elevated BUN level (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01–1.11; p=0.014) and initial Forrest classification (Forrest IA or IB vs. IIA or IIB; OR, 5.13; 95% CI, 1.11–23.74; p=0.036) (Table 4).
Clinical characteristics according to the location of the bleeding lesion
Differences in the treatment outcomes depending on the location of bleeding were mainly observed for upper GI bleeding. Among the upper GI ulcer bleeding cases that occurred continuously despite conventional hemostasis, the duodenum was the most common location (75.8%). The clinical success rates in the fundus and cardia were significantly lower than those at other locations in the upper GI bleeding group (p=0.033). There were three cases of lower GI bleeding in the sigmoid colon and eight in the rectum; however, there was no significant difference in clinical outcomes depending on the location of lower GI bleeding. These data are presented in Table 5.
DISCUSSION
In this retrospective, multicenter study, the use of hemostatic powder (UI-EWD) resulted in high technical and clinical success rates of 97% each. However, the early, delayed, and refractory rebleeding rates were relatively high. In the multivariate analysis, the factors contributing to early rebleeding were the initial BUN level and the first bleeding pattern of Forrest classification IA or IB. This multicenter study is significant as it reflects the current clinical practice of hemostatic powder treatment, which has not been retrospectively studied for GI bleeding in Korea.
In the current study, the clinical success rate of UI-EWD for upper GI bleeding was 96.8%, consistent with a previous single-center study of UI-EWD (96.4%).6 These results were higher than the clinical success rate of TC-325 (91%) and similar to that of polysaccharide hemostatic powder (96.7%).3,8 In our study, the early rebleeding rate of upper GI bleeding was 20.2%, which was similar to the early rebleeding rate of 20.0% in TC-325. However, delayed and refractory rebleeding showed higher rates than those reported in previous studies.3,8 Taken together, using UI-EWD for upper GI bleeding is effective for immediate hemostasis; however, there remains a high risk of rebleeding.
In our study, the factors affecting early rebleeding were the initial BUN level and initial active bleeding, presenting as Forrest classification IA or IB. According to Sung et al.,14 Forrest IA ulcers have a higher rebleeding rate than IB ulcers. Rodríguez de Santiago et al.15 reported spurting bleeding as an independent failure predictor. The Forrest classification, an indicator traditionally used to identify patients with a high risk of rebleeding,20 was also a significant predictor of rebleeding in our study. Similarly, an initially elevated BUN level was a predictor of early rebleeding in our study. Since BUN is also included in the Glasgow-Blatchford bleeding score (GBS), used to classify patients with GI bleeding risk,21,22 attention should be paid to initially high BUN level, even after hemostatic powder therapy. According to Chang et al.,23 among the AIMS65, GBS, and Rockall scores, scoring systems that classify patients with upper GI bleeding into high- and low-risk categories could not satisfactorily predict the rebleeding rate in patients with upper GI bleeding. Therefore, our study could be considered successful as it identified predictive factors for rebleeding.
In our study, the clinical success rate in the tumor group was 88.5%, which was lower than that in the ulcer group. Regarding the reason for the low success rate of tumor bleeding in our study, the extent of the tumor was relatively large in the three patients who did not achieve immediate hemostasis. One patient had a bleeding tumor size of 60 mm, and the size in the other patient was difficult to estimate owing to oozing bleeding in the entire gastric mucosa. In addition, of the three patients who failed to achieve immediate hemostasis, two had pancreatic cancer and one had gastric cancer; therefore, these patients’ general condition might be relatively poor. Tumor size and poor general condition may have influenced the clinical success rate. However, our study included only 26 patients with tumor bleeding, which might have resulted in lower success rates than previous studies.16 Technical problems in UI-EWD therapy happened when spraying the hemostatic powder in several cases. The powder did not emerge because of catheter malfunction and a new catheter was used. Additionally, because the hemostatic powder hardens when it comes into contact with water, only a minimal portion was sprayed.
One adverse event, perforation, was recorded. A 68-year-old woman with hypertension and esophageal squamous cell carcinoma previously treated with epinephrine injections for tumor bleeding was diagnosed with esophageal perforation after UI-EWD therapy. The patient was then surgically stabilized. Previous studies have also reported very low adverse event rates in patients treated with hemostatic powder3-6,8,9,14-16; therefore, inexperienced endoscopists may use them relatively safely.
Our study has several limitations. First, it was a retrospective study. Although we included various bleeding lesions, a detailed analysis of the bleeding lesions could not be performed. In particular, we could not compare the success rate of hemostasis with that of conventional hemostasis methods without UI-EWD. Second, the use of UI-EWD was dependent on the endoscopist’s decision; therefore, there might have been endoscopist subjective opinions, and the efficacy of UI-EWD might have been overestimated. Identifying whether the hemostatic powder was used because bleeding continued after primary hemostasis or was used as a preventive measure even after the bleeding stopped was challenging. Third, the short study period and the relatively small sample size may have reduced the reliability of our results. Further large-scale, multicenter studies are warranted.
In conclusion, in this retrospective, multicenter study, UI-EWD showed high technical and clinical success rates in patients with upper and lower GI bleeding after the initial standard hemostasis. However, the rebleeding rate was relatively high, even after UI-EWD therapy. Thus, early rebleeding should be considered in patients with initially high BUN levels and active bleeding according to the Forrest classifications IA and IB.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
This study was supported by a grant from the Korean Gastrointestinal Endoscopy Research Foundation (2022 Investigation Grant).
Author Contributions
Conceptualization: SIS, BIL; Data curation: ZHL, DSM, SHK, HHL, SK; Formal analysis: ZHL, SIS; Funding acquisition: SIS; Investigation: SIS, BIL; Methodology: SHK, HHL; Project Administration: SIS; Resources: ZHL, DSM, SHK, HHL, SK; Software: ZHL, SIS; Supervision: SIS; Validation: BIL; Visualization: ZHL, SIS; Writing–original draft: ZHL, DSM, SHK, HHL, SK, SIS; Writing–review & editing: SIS, DSM, SHK, HHL, SK, BIL.