Endoscopic Treatment of Refractory Gastroesohageal Reflux Disease
Article information
Abstract
Though efficient acid suppression with proton pump inhibitors (PPIs) remains the mainstay of treatment of gastroesophageal reflux disease (GERD), some of the patients showed refractory response to PPIs, necessitating further intervention. After increasing dose of PPIs and other kinds of pharmacological intervention adopting prokinetics or others, variable endoscopic treatments are introduced for the treatment of these refractory cases. The detailed introduction regarding endoscopic treatment for GERD is forwarded in this review article. Implantation of reabsorbable or synthetic materials in the distal esophagus was tried in vain and is expelled from the market due to limited efficacy and serious complication. Radiofrequency energy delivery (Stretta) and transoral incisionless fundoplication (EsophyX) are actively tried currently.
INTRODUCTION
Gastroesophageal reflux disease (GERD) is the most common gastrointestinal disorder; its prevalence is around 10% to 20% in the Western countries, but rather lower in Asia.1,2 Acid suppression with proton pump inhibitors (PPIs) remains the mainstay of treatment,3 but about 10% to 40% of the patients with GERD fail to respond to this treatment, causing refractoriness to PPI therapy. Refractory GERD is defined as <50% improvement in reflux symptoms including heartburn in spite of at least 12 weeks of double dose PPI therapy.4 The mechanisms of refractory GERD are variable, including motility disorder of the esophagus, functional heartburn, persistent reflux of acid, or nonacid material, esophageal hypersensitivity, and psychological comorbidity. For patients with typical reflux symptoms and inadequate response to PPIs, in whom abnormal esophageal acid exposure or positive symptom reflux association are demonstrated, surgical fundoplication can be a relatively safe and highly effective procedure.4 Remission rate was reported about 85% to 93.5%, whereas the complication rate (0.06% to 1.3%) and mortality rate (0% to 0.08%) are relatively low.5-7 However, postoperative side effects such as persistent dysphagia, inability to belch, increased bloating and flatulence are common and may persist for more than 6 month.5 Recently, various endoscopic treatments have been tried. These endoscopic treatments are largely divided into three categories as follows; injection or implantation techniques, radiofrequency (RF) ablation, and endoluminal gastroesophageal plication. That is, Stretta procedure and the EsophyX transoral incisionless fundoplication (TIF) are currently available for human use for refractory GERD. The beneficial mechanisms after these procedures are alteration in the cardia compliance, decrease in transient lower esophageal sphincter (LES) relaxations, increase from the baseline LES length, and decrease in the diameter of either the distal esophagus or gastroesophageal junction (GEJ).8
THE STRETTA PROCEDURE (RADIOFREQUENCY ENERGY DELIVERY)
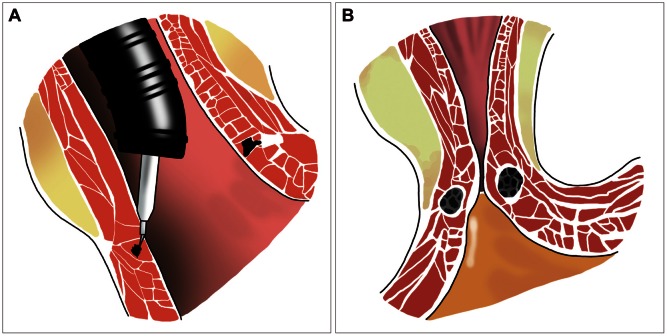
The radiofrequency (RFE) delivery was approved by Food and Drug Administration (FDA) 2000 (Stretta; Curon Medical Inc., Sunnyvale, CA, USA). The Stretta applies thermal RFE at the level of the LES and gastric cardia, then the RFE causes a limited coagulative necrosis of the tissue, which is finally healed by fibrosis.8,9 This procedure can be done in the outpatient setting with conscious sedation and procedure time of about 40 to 45 minutes. The Stretta system consists of RF control module and a flexible stretta catheter. The catheter consists of a 20 F soft bougie tip and a balloon, which opens in a surrounding basket. The four electrodes provide 60 to 300 J of RF energy to each needle, heating the surrounding muscle tissue to the target temperature between 65℃ to 85℃. Continuous irrigation of the esophageal mucosa and surface temperature monitoring is utilized to prevent thermal mucosal injury (Fig. 1A).9,10 RF energy induces shrinkage of the esophageal collagen fibrils, then the LES become more tightened (Fig. 1B), preventing acid reflux from the stomach.11 Furthermore, remodeling of the stretch fibers located in the cardia occurs and the vagal afferent signals to the brainstem triggering transient LES relaxations are finally interrupted. Therefore, it could be quite effective in decreasing esophageal sensitivity to acid.12 Several controlled clinical studies have showed clinically significant improvement in esophageal symptoms as manifested by the mean score decreasing from 2.7 at baseline to 0.6 at 48 months and 68.7% of patients showing complete resolution of symptoms, improved quality of life (QOL) scores, significantly decreased PPI use from 100% to 13.75%, decreased esophageal acid exposure from 44.37 to 28.53 on Johnson-De-Meester score, and increased LES pressure from 16.54 to 20.24 mm Hg.13,14
(A) The stretta system. The four electrodes provide 60 to 300 J of radiofrequency energy to each needle, heating the surrounding muscle tissue. (B) The lower esophageal sphincter is tightened after radiofrequency ablation.
Therefore, the Stretta procedure can be helpful in patients with refractory GERD. The other advantage is that the procedure is relatively safe and easy. The most common complications are gastroparesis and ulcerative esophagitis, which are rare. Transient epigastric pain or chest pain, low grade fever, dysphagia, or odynophagia was also reported.14,15 However, there are limited data yet on the effectiveness of this procedure, necessitating further extended clinical application to warrant safety and effectiveness.
THE INJECTION OR IMPLANTATION TECHNIQUES
The principle of endoscopic injection methods consists of the endoscopic implantation of nonreabsorbable or synthetic material in the distal esophagus, for which various bulking methods are used as follows.
Enterynx (etylene vinyl alcohol copolymer)
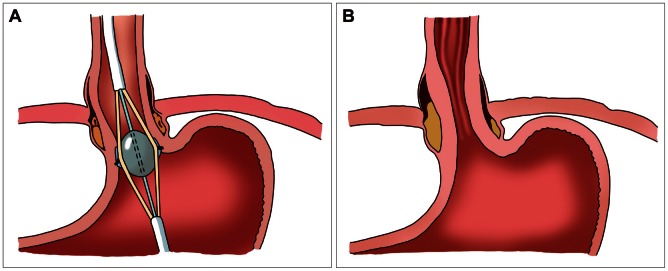
Enterynx (Boston Scientific, Natick, MA, USA) was approved by the FDA in April 2003.8 It is consisted of a biocompatible polymer (8% ethylene vinyl alcohol copolymer), mixed with a radio-opaque contrast agent (30% tantalum powder) and dissolved in an organic liquid carrier (dimethyl sulfoxide).16 The procedure can be performed under conscious sedation. The needle catheter, 23 to 25 gauze, is inserted through the working channel of the endoscope and Enterynxis is injected into the muscular and submucosal layers at a point about 1 to 2 mm caudal to the Z-line. Four quadrant injections of 1 to 2 cc each are made at the same level. After injection, Enterynx is rapidly diffused into the tissue, resulting in the precipitation of the polymer as a spongy mass (Fig. 2A).16,17 Although Enterynx does not affect LES pressure, it may augment the gastroesophageal reflux barrier zone. The distensibility and shape of esophagogastric junction (EGJ) is changed (Fig. 2B). The fibrous encapsulation might lengthen the LES, potentially leading to an elevated threshold for transient LES relaxation. The 53% to 80% of the patients can cease PPI and there was significant improvement of symptoms and QOL in over 50% of patient. However, normalization of pH was seen only in about 30% of the patients.18,19 Though seemingly effective, the procedure was notorious for severe complications such as esophageal abscess,20 pneumomediastinum,21 esopohageal perforation, renal failure, esophageal stenosis, and even death due to intra-aortic injection,22 after which this procedure was withdrawn in 2005.
Plexiglas (polymethylmethacylate)
Plexiglas (Rohm GmbH & Co., Darmstadt, Germany) is a suspension of polymethylmethacylate microspheres in gelatin solution.8,15 It is injected into six proximal areas of EGJ. After injection, the gelatin is phagocytized by macrophages within 3 months and replaced by fibroblasts and collagen fibers. The microspheres are encapsulated by connective tissue.23 Significant decreases in either the symptoms or the mean total time with an esophageal pH of less than 4 were achieved. However, there was only one publication about the use of Plexiglas in the treatment of GERD.23
Gatekeeper TM system (dehydrated hydrogel)
The Gatekeeper Reflux Repair system (Medtronic, Shoreview, MN, USA) was approved for clinical use in the European Union in May 2003. However, Medtronic ceased its development and voluntarily withdrew the device from the market.8 Through the mechanism that expandable polycrylonitrile-based hydrogel prostheses injected into the distal esophageal submucosa augments the LES and creates a reflux barrier, the reflux symptoms were reduced and a normal pH level was achieved, accompanied by medial increase of LES pressure, in 40% of the patients.15 However, the procedure related serious complications, such as esophageal perforation, pulmonary infiltrate and severe chest pain, limited further extension of its use.22 This method was voluntarily withdrawn in 2006 due to quite disappointing results.
ENDOLUMINAL GASTROESOPHAGEAL PLICATION
Several endoluminal devices have been developed for creating a suture or plication of the gastric folds at GEJ, reproducing a valve mechanism mimicking surgical fundoplication.
Mucosal suture plication
EndoCinch (Bard Inc., Billerica, MA, USA), an endoscopic suturing system, was approved by FDA in 2000.8 The device produces suction of tissue just below Z-line, then needle with preloaded suture is advanced, followed by cinching/cutting catheter advanced to the tissue.24 This procedure can be performed in the outpatient setting. It was effective in short-term follow-up period and the complication rate was relatively low. However, sutures were significantly lost within the 6-month follow-up period, thus necessitating reprocedure in about 25% of the patients. Most patients returned to baseline in long-term follow-up because mucosa-to mucosa plication did not lead to serosal adhesion.25,26
Full-thickness plication
To overcome the limitation of mucosal plication, Full-thickness plication devices are developed. NDO plicator system (NDO Surgical Inc., Mansfield, MA, USA), consisting of endoscopic tissue retractor and suturing system, was approved by FDA in April 2003.8 A gastroscope equipped with the plication device is introduced through an over-tube. The helical tissue retractor retracts the the stomach wall, approximately 1.5 cm below the cardia, then the instruments arms are closed, creating a serosa-serosa placation.27 The QOL of patients were improved by more than 50%, and the use of PPI was significantly decreased in 50% to 80%, accompanied with decreased esophageal acid exposure.28 On the contrary to these achievements, normalization of pH was observed in only 14% to 30% of the patients, and LES pressure measured by manometry was generally unchanged.25-28 Fever, abdominal pain, pharyngitis were reported. Serious complications such as perforation, pneumothorax, pneumoperitoneum, pneumediastinum, adhesions, pleural effusion, and aspiration leading to death have been also reported.15,26 The device has become unavailable since 2008.
TIF
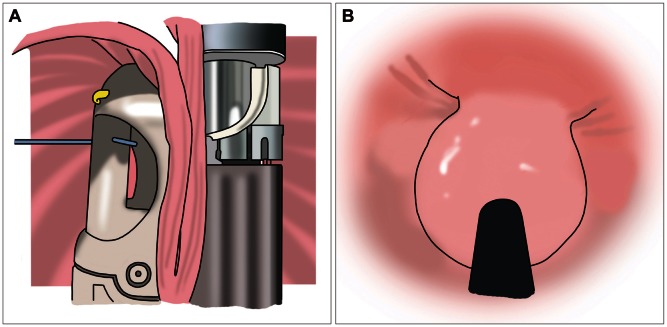
TIF (EsophayX; EndoGastric Solutions Inc. Redmond, WA, USA) is the latest device for endoscopic treatment of refractory GERD. The procedure can be performed under general anesthesia. One physician controls the device and another physician operates the endoscope. The device retracts the gastric cardia, and creates full-thickness serosa to serosa plication and valve (Fig. 3A). The valve produced by EsophyX is 3 to 5 cm in length, 200 to 300 µM in circumference (Fig. 3B) and plays the role of gastroesohagel sphincter.27 It can be a less invasive alternative treatment to laparoscopic fundoplication for refractory GERD patients. Over 70% of the patients ceased or reduced PPIs use.29-31 The reflux symptoms improved significantly, which was maintained up to 3 years' follow-up.29 Also resolution of hiatal hernia and reflux esophagitis were reported in over 55% to 97% of patients.30 Reflux characteristics in terms of acid exposure, number of refluxates, and DeMeester scores improved significantly and were normalized in 61% to 89% of patients in a retrospective study.32 However, some studies reported that EsophyX was less effective than surgery and laparoscopic Nissen fundoplication have been reported effective in EsophyX failure cases.33 Therefore, the potential value of this technique should be further evaluated in controlled prospective trials. The procedure is relatively safe; the overall complication rate is 3% to 10%. Serious complication, such as esophageal perforation, intralumnal bleeding and mediastinal abscess, can occur but are rare31 (<5%) and no procedure related deaths have been reported.29
CONCLUSIONS
Though endoscopic treatment is a reasonable alternative treatment for refractory GERD, there still remains room for improvement. TIF EsophyX is promising, but long-term follow-up and prospective studies are needed.
Notes
The authors have no financial conflicts of interest.