Technical Advances in Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition for Pancreatic Cancers: How Can We Get the Best Results with EUS-Guided Fine Needle Aspiration?
Article information
Abstract
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is one of the least invasive and most effective modality in diagnosing pancreatic adenocarcinoma in solid pancreatic lesions, with a higher diagnostic accuracy than cystic tumors. EUS-FNA has been shown to detect tumors less than 3 mm, due to high spatial resolution allowing the detection of very small lesions and vascular invasion, particularly in the pancreatic head and neck, which may not be detected on transverse computed tomography. Furthermore, this minimally invasive procedure is often ideal in the endoscopic procurement of tissue in patients with unresectable tumors. While EUS-FNA has been increasingly used as a diagnostic tool, most studies have collectively looked at all primary pancreatic solid lesions, including lymphomas and pancreatic neuroendocrine neoplasms, whereas very few studies have examined the diagnostic utility of EUS-FNA of pancreatic ductal carcinoma only. As with any novel and advanced endoscopic procedure that may incorporate several practices and approaches, endoscopists have adopted diverse techniques to improve the tissue procurement practice and increase diagnostic accuracy. In this article, we present a review of literature to date and discuss currently practiced EUS-FNA technique, including indications, technical details, equipment, patient selection, and diagnostic accuracy.
INTRODUCTION
Despite advancements in medical and surgical therapy in the past few decades, pancreatic cancer continues to be a devastating disease with a dismal prognosis. Unfortunately, the incidence of pancreatic adenocarcinoma is rising and it remains one of the leading causes of cancer-related deaths worldwide.1,2 Multiple epidemiologic studies have shown the 5-year survival rate to be less than 5%.1,3 This is in part due to the delayed diagnosis of this cancer in individuals often because of the late onset of symptoms. At initial imaging evaluation, about 80% of patients will already have locally advanced disease or advanced metastasis, thus precluding the possibility of a curative surgical resection.4 Of the 20% of patients that appear resectable and index evaluation, less than half end up undergoing curative surgical resection because of regional tumor spreading at the time of surgery.4 Therefore, early and accurate diagnosis is crucial to provide patients with the necessary time and information to make appropriate treatment decisions and improve their prognosis.
Endoscopic ultrasound (EUS) has been shown to be a valuable imaging tool for the detection of pancreatic lesions. However, it is the capability to perform fine needle aspiration (FNA) and provide a concurrent tissue diagnosis at the time of EUS that has made it an essential tool in the diagnostic algorithm for various visceral malignancies, especially solid pancreatic lesions. Since the first report of EUS-FNA of the pancreas by Vilmann et al.5 in 1992, EUS-FNA has become the standard of care for tissue diagnosis in the pancreas because it is a safe, effective, and accurate procedure.6 Various decisions and facets go into performing successful EUS-FNA such as selection of needle size and type, location of the targeted lesion, availability of onsite cytopathology, use of accessories such as stylets and suction, endoscopic technique, and utilization of novel imaging enhancing techniques like elastography and contrast-harmonic EUS. This review will summarize the current literature regarding these various aspects of EUS-FNA and how to optimize tissue acquisition in the pancreas.
DIAGNOSTIC EUS
The four main objectives of EUS in managing pancreatic lesions include detection, staging, determining surgical resectability, and making a confirmatory tissue diagnosis. The first three goals do not require tissue acquisition. The proximity of the echoendoscope to the pancreas allows for exceptional imaging of the head, neck, and uncinate process from the duodenum and the body and tail from the stomach. Multiple studies have shown EUS to be both sensitive and accurate in staging pancreatic lesions. Also the high negative-predictive value of EUS when evaluating pancreatic lesions makes it a reasonable rule out test for malignancy, which can be helpful to clinicians in situations of unclear cross-sectional imaging.7,8 Although some of the superiority that EUS enjoyed for many years over standard computerized axial tomography (computed tomography, CT) has diminished due to improved cross-sectional imaging technology (i.e., helical CT), many experts still consider it to be the single best test for evaluation of pancreatic lesions.8,9
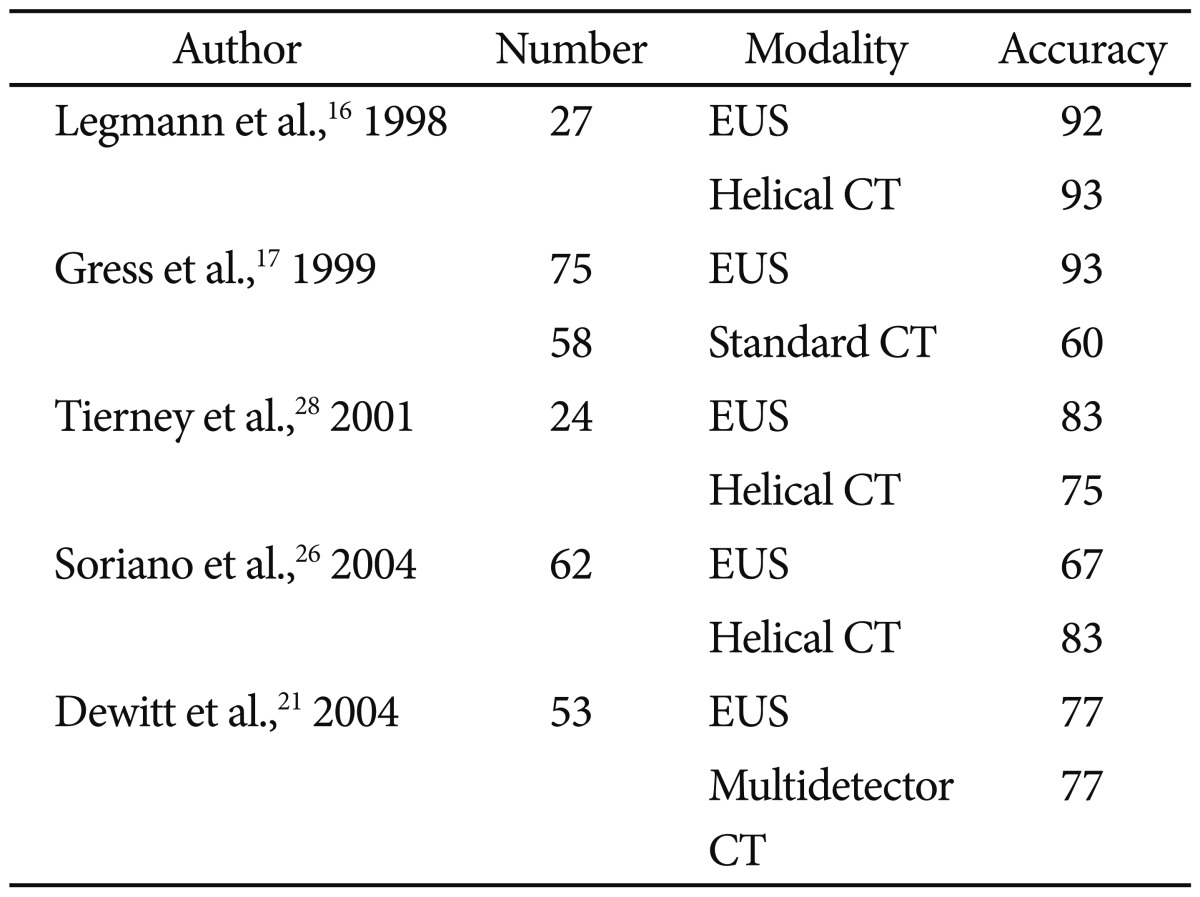
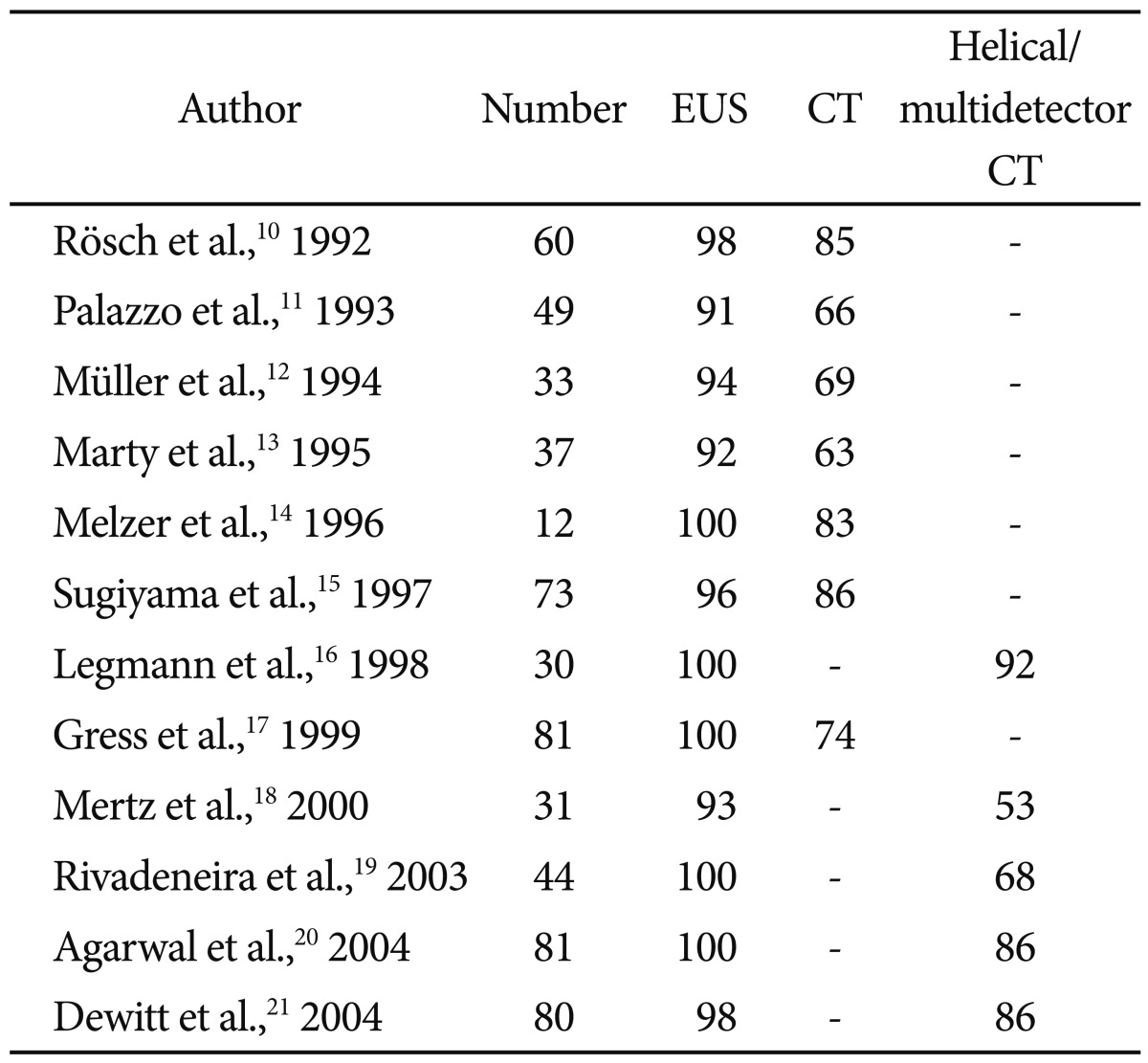
In terms of pancreatic tumor detection, EUS has been shown in prior to studies to be superior to cross-sectional imaging (Table 1).10-21 Especially for smaller lesions (<3 cm), EUS holds the advantage over CT and transabdominal ultrasound.22 Although some authors question whether the dominance of EUS over cross-sectional imaging given modern advances in radiographic imaging, a recent systemic review reinforced the conventional data and popular consensus.23
Sensitivity of Endoscopic Ultrasound Compared to Cross-Sectional Imaging for Detecting Pancreatic Lesions
Once a lesion has been discovered, staging that lesion and determining whether it is surgically resectable is of utmost importance to establish a prognosis and means of treatment. As with EUS sensitivity, earlier studies of comparing EUS to standard CT showed a clear advantage of EUS in staging tumors and determining resectablity (Tables 2, 3).10-12,16,17,19,21,24-28 Generally EUS consistently performed better than standard CT for staging and determining tumor extension, however the results are less consistent when compared to helical or multidetector CT.29 In one study, EUS was most accurate at assessing tumor size and lymph node involvement, while helical CT was more precise regarding overall staging, resectability, vascular invasion, distant metastasis, and locoregional extension.26 Systemic reviews have also not been able to show a true frontrunner regarding this issue, thus many authors recommend that the most prudent use of these modalities is as complementary rather than competing tools.8,23
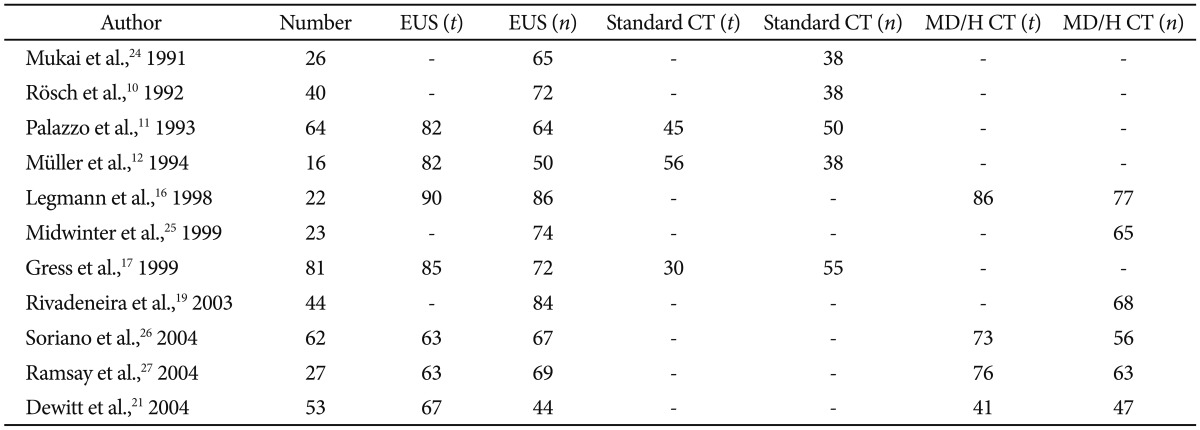
Accuracy of Endoscopic Ultrasound Staging Pancreatic Cancer (T and N) Compared to Cross-Sectional Imaging
EUS-FNA
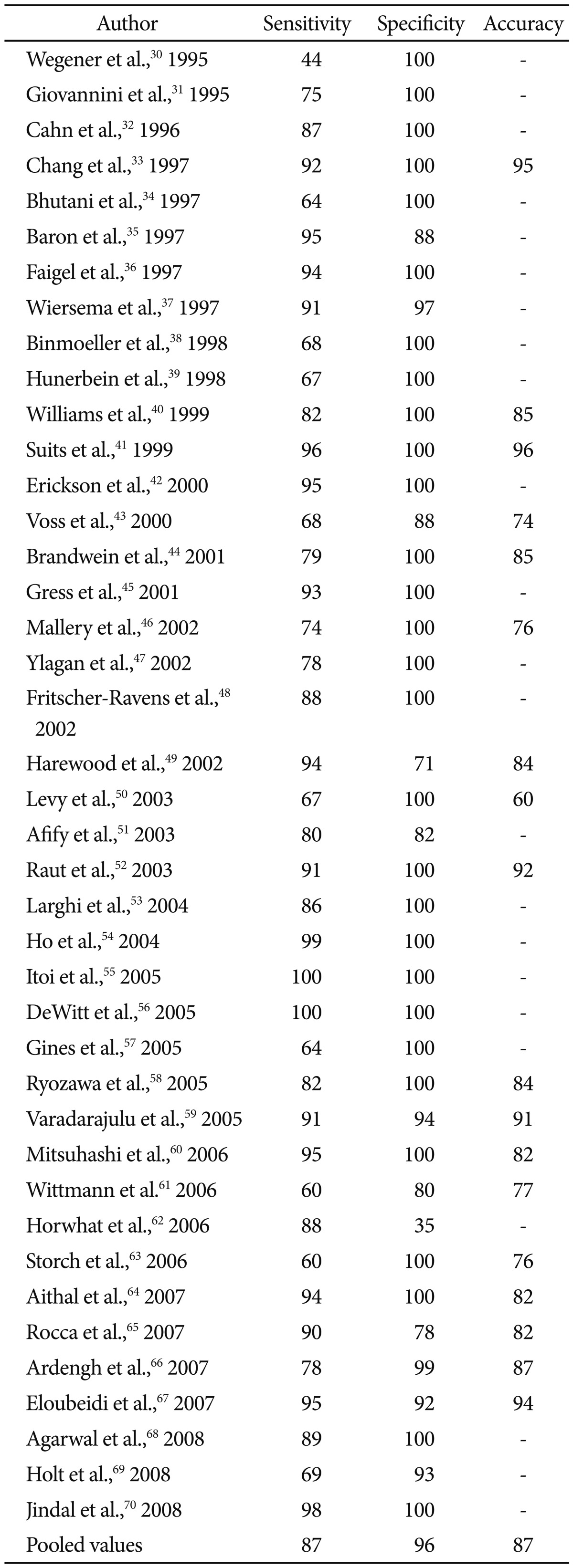
Although EUS has clearly been established as the most sensitive modality for pancreatic lesions, distinguishing malignant from benign etiologies can be difficult in certain clinical scenarios such as chronic pancreatitis. For this reason, establishing a tissue diagnosis using the cytopathology and histology obtained from EUS-FNA has become both paramount and essential to this technique. EUS-FNA has become widely accepted as a safe, effective, and consistent method of diagnosing pancreatic cancer.8 A recent meta-analysis evaluated 41 studies with a total of 4,766 patients having undergone EUSFNA for pancreatic lesions and reported the pooled accuracy to be 86.8% and specificity 95.8% (Table 4).30-70 This study also found that the accuracy of EUS-FNA is improving over time; in subgroup analysis, EUS-FNA accuracy was higher during 2001 to 2009 than between 1995 to 2000. Another systemic review including studies from the past 10 years confirmed this notion as they reported pooled sensitivity and specificity rates of 92 and 96%.6 The etiology of this trend is likely multifactorial due to the natural learning curve of this procedure in the endoscopic community, better instruments, and the increased availability of specialized training for EUS-providers.
EUS-FNA VERSUS OTHER TISSUE SAMPLING MODALITIES
Compared to alternate tissue-acquisition techniques such as percutaneous CT-guided biopsy and endoscopic retrogrograde cholangiopancreatography (ERCP) brush cytology, EUS-FNA holds an advantage of being more sensitive and less invasive. It is well established that the sensitivity of ERCP brush cytology is quite low and ranges anywhere between 30% to 85%.71 Therefore, EUS-FNA has supplanted ERCP brush cytology as the primary method of pancreatic tissue acquisition especially in patients without obstructive jaundice. When compared to CT-guided biopsy, EUS-FNA has both the logistical and economic advantage being able to image and obtain tissue during a single-session as opposed to two separate occurrences. Head to head trials have also supported the increased sensitivity of EUS-FNA compared to CT-guided biopsy.62 Also there is a concern that CT-guided biopsy may confer a higher risk of peritoneal tumor seeding compared to EUS-FNA, which has been shown in retrospective studies to be as high as 16.3% compared to 2.2%.72 EUS-FNA has also been shown to be more sensitive for detecting and sampling malignant ascites compared to CT, therefore furthering its diagnostic advantage over percutaneous sampling techniques.73
EUS-FNA has also proved to be a safe and effective salvage biopsy maneuver in cases of nondiagnostic ERCP brush cytology and CT-guided biopsy. In the study by Harewood and Wiersema49 185 patients with pancreatic masses were assessed by CT-guided biopsy or ERCP brush cytology prior to going to EUS-FNA. Of the 58 patients with negative CT-guided biopsies and 36 patients with negative ERCP brush cytology, subsequent EUS-FNA detected malignancy in 90% and 94% of patients.49 The high sensitivity and accuracy of EUS-FNA after previously negative tissue sampling procedures has been confirmed in other studies as well, which boast sensitivity and accuracy rates of 93% and 88%.45,74
EUS-FNA SAMPLING: TECHNIQUES AND ACCESSORIES
Positioning and technique
In general, EUS-FNA is performed best when the echoendoscope is in a stable position with a straight tip, thus allowing for easy passage of the FNA needle. This is usually achieved more often in the transesophageal and transgastric position as opposed to the transduodenal. When targeting the uncinate process or pancreatic neck from the duodenal bulb, the tip of the echoendoscope is flexed, thus making needle passage more difficult.75 Troubleshooting this issue by maneuvering the echoendoscope into the long position may solve the problem, however at the expense of a more precarious scope position. The other option to deal with difficult passage of the needle in torqued or flexed positions is to choose a smaller gauge (G) such as the 25 G needles to allow for easier passage.
It is important during EUS-FNA for the endoscopist to actively attempt to sample multiple sections of a pancreatic lesion rather than mechanically penetrate only one tissue tract. Because neoplastic lesions can be heterogenous in nature, with necrotic, acellular centers, it is important to target multiple areas of the lesion especially the periphery to improve cellular yield.75 A recently described fanning-technique has become accepted amongst endosonographers as one study has shown it to improve first pass diagnostic rates by almost 30%.76 The idea is to reposition the needle angle using the dials and elevator intermittently to successively sample from multiple areas of the lesion rather than one singular angle. It is thought that the fanning technique works not only by successively sampling new tracts of tissue, but limiting the amount of blood and artifact from previous tract sites.75,76
One recurrent question endosonographers face is the least number of passes that is adequate to sample a pancreatic lesion. In cases where onsite cytopathology is available, this question becomes much easier to answer because there is real-time feedback on FNA yield. However, in cases without that luxury, the risk of causing more cellular injury and possible complications must be weighed against the benefit of improving diagnostic accuracy. As of now, there is no generalized consensus about the optimal number of passes or sampling techniques for EUS-FNA of pancreatic lesions. Various studies have estimated the ideal number of needle passes in the pancreas without onsite cytopathology to be between 3 and 7.77-79 Wallace et al.77 recommended 3 needle passes each with back and forth motions of about 30 seconds each to adequately detect pancreatic malignancy.75 Pellise Urquiza et al.78 found that the diagnostic plateau for EUS-FNA is reached after the fourth pass. Leblanc et al.79 found that the diagnostic sensitivity of 7 and 5 needle passes in the pancreas and lymph nodes to be about 83% and 77%. While there is no consensus, based on the available data, if no onsite cytopathology is available some authors recommend at least 5 to 6 passes in the pancreas and 2 to 3 passes in lymph nodes for adequate EUS-FNA sensitivity.75
Choosing the needle
Unfortunately, there is no single EUS-FNA needle available that is perfect for every pancreatic lesion. Therefore, the endoscopist must use their experience and clinical judgment to decide on the appropriate needle type. Generally when making this decision, they should consider which needle will optimize cellular yield, minimize complications, and specimen contamination, and the need for needle flexibility based on the lesion's location in the pancreas. As stated before, transduodenal puncture to sample pancreatic head, neck, and uncinate lesions may demand more pliable needles due to the flexed tip of the echoendoscope.
As of now, there are three needle G (19, 22, 25 G) available for EUS-FNA. Multiple prospective studies, including four randomized-controlled trials (RCTs) have been performed to compare the diagnostic yield and accuracy of these needles (Table 5).80-84 Three of the RCTs compare 22 and 25 G needles without any statistically significant differences in diagnostic accuracy, although there was a trend towards significance when using 25 G needles in pancreatic head/uncinate lesions.81,82 One prospective study by Sakamoto et al.84 showed a clear benefit of the 25 G over the 22 G for uncinate processes with reported diagnostic accuracies of 100% versus 33%. These studies also revealed that while the 19 G needle may improve cellular yield compared to the smaller needles, it comes at the expense of decreased efficacy for transduodenal lesions. A recent meta-analysis on the issue found that 25 G needles may have a slight benefit over 22 G in terms of specimen adequacy, however this did not translate into significantly higher diagnostic accuracy or fewer complications.85 Therefore, the choice between 25 and 22 G needle for pancreatic body and tail masses may be left up to the endoscopist's preference with the caveat that 25 G is probably superior for pancreatic head lesions. On the other hand, 19 G needles may provide higher cellular yields and potentially provide histologic sample, but are not generally effective in pancreatic head lesions.
Histology: EUS-fine needle biopsy
Despite the impressive outcomes achieved with EUS-FNA, there are some limitations of obtaining tissue via aspiration for cytology. As mentioned earlier, one limitation is the unclear number of passes required to achieve an adequate sample in the absence of an onsite cytopathologist. This issue is amplified in settings of pancreatic tissue fibrosis and distortion such as chronic pancreatitis when the cellular yield from EUS-FNA is diminished.59 Also, while usually 100 cells or less is the minimum number to obtain a pathologic diagnosis, some tests may require additional cells such as RNA extraction.8 Finally, cytology is devoid of tissue architecture, which may be necessary in certain situations to clarify a diagnosis such as lymphoma, autoimmune pancreatitis, and gastrointestinal stromal tumors.
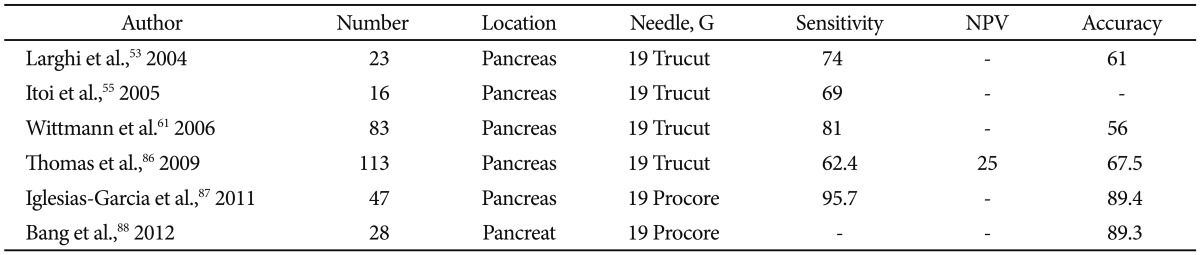
To address these concerns, various EUS-compatible core biopsy needles have been developed including the 19 G Trucut (Wilson Cook, Winston-Salem, NC, USA) and 19/22 G ProCore (Cook Endoscopy, Winston-Salem, NC, USA). These needles have various design mechanisms that allow for cutting and procurement of a solid core of architecture-preserved tissue. The actual benefit of EUS-guided fine needle biopsy (FNB) for pancreatic lesions is unclear. Various reports have quoted the accuracy rates of EUS-FNB in the pancreas to be between 61% and 89.4% (Table 6).53,55,61,86-88 The rigidity of 19 G Trucut needle proved to be a limitation for transduodenal sampling as Itoi et al.55 reported zero percent sensitivity for uncinate lesions. This limitation has been somewhat overcome with the development of more flexible core biopsy needles. One RCT comparing 22 G EUS-FNA and FNB needles for pancreatic lesions found no significant difference in diagnostic yield/adequacy, technical success, and complications.88 Some authors have shown that the combination of both modalities may improve both sensitivity and accuracy in assessing pancreatic lesions.61 Thus, as of now, there is no clear indication that EUS-FNB is preferred over EUS-FNA for pancreatic lesions unless histologic analysis is required.
Using suction
Conventionally, the use of suction on the FNA needle system had been standard in the endoscopic world because of the intuitive notion that it would increase cellular yield. However, although this has shown to be true in various studies, it comes at a price of decreasing the quality of the specimen due to increased bloodiness. This has been shown in two of the three RCTs performed on this topic (Table 7).77,89-91 Thus, while there is no consensus on the topic, many authors conclude that suction is of limited value during EUS-FNA pancreas because it reduces the quality of the specimen.8 However, in cases where cellular yield is low such as fibrotic lesions in chronic pancreatitis, use of suction may be appropriate to improve cellularity and diagnostic yield. In softer lesions, which may contain necrosis and blood, the use of suction is discouraged to minimize distortion of the cellular sample.
Using the stylet
In every EUS-FNA system, there is a stylet preloaded to the tip of the needle. The stylet is a rigid metal wire that runs through the length of the FNA needle to theoretically prevent any tissue plugs that might prevent adequate sample procurement of the target lesion as the FNA needle traverses visceral organs.75 Many endoscopists find the use of the stylet tedious and risky as it increases the chance for needle-stick injuries. Three RCTs have been performed looking at the diagnostic yield of performing EUS-FNA in the pancreas with or without and stylet.89,92-94 All three of these well-designed studies have shown consistent results in that the use of the stylet offers no advantage in terms of diagnostic yield, but does significantly increase the bloodiness of specimens. Therefore, there is no advantage to using the stylet when performing EUS-FNA of the pancreas.
Onsite cytopathology
Unfortunately, up to 32% of EUS-FNA specimens may be non-diagnostic for a variety of reasons including acellularity and specimen distortion from blood and artifact.95 Therefore, having a trained onsite cytotechnician or cytopathologist to actively assess the adequacy of samples during the procedure is very valuable, and unfortunately is a luxury that not all endosonographers can enjoy. Multiple studies have revealed that real-time onsite cytopathology increases the diagnostic yield and reduces the number of indeterminate or unsatisfactory samples from EUS-FNA (Table 8).96-98

Studies Evaluating the Role of Onsite Cytopathology at the Time of Endoscopic Ultrasound-Guided Fine Needle Aspiration
Alsohaibani et al.97 showed increased diagnostic yield of 22% even if a trained cytotechnician is preparing slides as opposed to an endoscopy nurse or technician. Other studies have also shown that onsite cytopathology can increase diagnostic yield by 15% to 29% along with reducing the number of required needles passes and possible complications associated with the procedure.99-101 The combination of increased diagnostic yield and fewer nondiagnostic specimens can translate into fewer needles used, shorter procedure times, fewer repeat procedures, and significant cost reduction. Indeed, the costbenefit of onsite cytopathology has been evaluated and estimated to be over $400,000 annually for a single institution in one study.78,95
SLIDE PREPARATION AND FIXATIVE SOLUTIONS
EUS-FNA specimens are prepared in two methods to create smear. For immediate interpretation, the specimens can be airdried and stained with Diff-Quik (Dade Diagnostics, Miami, FL, USA). Otherwise specimens can be fixed in an alcohol solution, which allows for nuclear preservation and eventually stained with Papanicolaou or H&E stains.75 If special stains are required, then the sample can be stored in liquid media and prepared for cell block, which involves centrifuge, fixation, sectioning, and staining with H&E. A newer liquid-based cytology (Thin prep; Cytyc Inc., Marlborough, MA, USA and SurePath; TriPath Inc., Burlington, NC, USA) is now available. The benefits of this technique are that it may mitigate human error during preparation, better preserve the cellular integrity, and display cells in a uniform monolayer dispersion.75 However, the liquid-based technology is costly and its accuracy has not been validated. Multiple studies have shown that traditional smear preparation exhibits a higher diagnostic accuracy (84% to 98% vs. 64% to 67%) when compared directly to liquid-based cytology. More studies are needed to evaluate the efficacy and usefulness of this preparation.
IMPROVING EUS-FNA: ELASTOGRAPHY, CONTRAST HARMONIC EUS, AND FLUORESCENCE IN SITU HYBRIDIZATION
Although EUS-FNA is a good test for evaluating pancreatic lesions, it is not perfect as evidenced by the pooled diagnostic accuracy of about 87% in a recent meta-analysis.29 Because making the diagnosis of pancreatic malignancy is so crucial to overall prognosis, various complementary imaging and analytic technologies have been developed to try and improve this limitation.
Two novel imaging technologies now available for EUS include elastography and contrast harmonic echo. Elastography capitalizes on the fact that diseased tissue such as malignancy can lead to altered mechanical properties of the tissue through remodeling, inflammation, and fibrosis.102 Thus, elastography can measure tissue stiffness and help differentiate benign versus malignant tissue without having to actually physically sample the lesion. Using the hue-histogram produced by the technology, which correlates with tissue elasticity, some studies have been able to show accuracy rates up to 89% to distinguish benign versus malignant pancreatic lesions and lymph nodes.103,104 A variable known as the strain ratio, which is a calculated quotient of the lesion's relative stiffness, has been shown to have a sensitivity and specificity of 100% and 92.9% in diagnosing pancreatic malignancies.105 Contrast-harmonic echo (CHE) is an imaging modality that enhances vascular imaging during EUS. Intravenous contrast agents containing gas-filled microbubbles are injected into peripheral veins thus allowing improved EUS visualization of the microvasculature, which may help diagnose malignant lesions. By evaluating the echogenicity and enhancement of pancreatic lesions during CHE, Fusaroli et al.106 were able to show improved detection (96%) and accuracy (82%) for pancreatic adenocarcinomas. While elastography and CHE are promising technologies, they are still relatively new and require further research to establish their role in evaluating pancreatic masses. As of now, they should be used as complementary tools to EUS-FNA in specialized centers that have experts trained in their usage.
Another enhancement on the interpretation side of EUSFNA has been the development of fluorescence in situ hybridization (FISH). FISH is a technique that can detect various chromosomal abnormalities by using specific, fluorescently-labeled DNA probes. Polysomy and trisomy FISH (aside from trisomy 7) have been shown to be independent predictors of malignancy with high specificities.107 Multiple studies have shown that in cases of indeterminate cytology, FISH can provide a diagnosis up to 79% of cases without compromising specificity.108,109 Further cost-effectiveness and efficacy studies are required to determine which in application of FISH is reasonable.
PITTFALS OF EUS-FNA: NONDIAGNOSTIC SAMPLING
Unfortunately, even the most experienced endoscopists cannot perform EUS-FNA with 100% sensitivity due to the inherent limitations of this technology. Lower diagnostic yields are inevitable in certain clinical diagnoses like chronic pancreatitis, which complicates sampling due to increased parenchymal lobularity and calcification.59 Also transduodenal EUSFNA can be challenging due to the position of the endoscope, passage of the needle, and visualization of the target lesion. Cytology samples, although adequate, may not be definitive in many cases. And finally, the lack of an onsite cytopathologist, often in the community setting, puts endosonographers at an immediate disadvantage in terms of procuring a diagnostic sample.
There is no universally accepted guideline on how to manage patients with suspected pancreatic cancer, but negative FNA sampling. The management options include repeating the EUS-FNA, attempting CT-guided biopsy, or proceeding to surgical exploration. In healthy, surgically-resectable patients, the decision to go to surgery is relatively straightforward. However, in less healthy/older patients with an unclear clinical picture or margin of tumor, that may be poor surgical candidates, the situation becomes more challenging. Generally, performing CT-guided biopsy is avoided because of the risk of peritoneal tumor seeding.72 Therefore, in these cases, repeating the EUS-FNA may be the most prudent approach, with reported diagnostic rates of 61% to 84%.110-112
For newly trained endosonographers practicing in settings without onsite cytopathology, and thus are susceptible to lower EUS-FNA diagnostic yields, consideration should be given to learning how to self-interpret cytopathology specimens. In these cases, EUS-FNA method should be optimized including performing the appropriate number of passes (at least six to seven in the pancreas and three in lymph nodes) along with the fanning technique to minimize insufficient specimens.75 Use of the 19 G core biopsy needle may also improve cellular yield. It is important for proceduralists performing EUS-FNA to understand the indications and relevant literature behind the tools they are using to ensure the best outcomes of the procedure and minimize nondiagnostic sampling.
CONCLUSIONS
EUS-FNA has become a necessary tool for the complete and accurate evaluation of pancreatic lesions. Although it may be the single best test, starting with cross-sectional imaging to initially evaluate a pancreatic lesion will complement further EUS-FNA assessment. EUS-FNA has established itself as the least invasive, safest, and most effective tool for tissue acquisition in the pancreas. It should be performed by trained endosonographers using the proper tools and techniques. Generally, the use of suction, stylets, and core biopsy needles has not proven to improve diagnostic accuracy. However, utilization of onsite cytopathology, the fanning technique, and appropriate needle gauge depending on the lesion's location improves outcomes. Research is ongoing on how to improve EUS-FNA in terms of novel imaging (elastography, CHE) and sample interpretation (FISH). EUS-FNA has established itself as the first-line procedure for tissue acquisition in the pancreas and will continue to improve as further research is performed to improve its outcomes.
Notes
Dr. Michel Kahaleh has received grant support from Boston Scientific, Fujinon, EMcison, Xlumena Inc., MaunaKea, W.L. Gore, Cook Endoscopy, Aspire Bariatrics, GIDynamics, and MI Tech. He is a consultant for Xlumena Inc. and Boston Scientific.