Advanced Imaging Technologies for the Detection of Dysplasia and Early Cancer in Barrett Esophagus
Article information
Abstract
Advanced esophageal adenocarcinomas arising from Barrett esophagus (BE) are tumors with an increasing incidence and poor prognosis. The aim of endoscopic surveillance of BE is to detect dysplasia, particularly high-grade dysplasia and intramucosal cancers that can subsequently be treated endoscopically before progression to invasive cancer with lymph node metastases. Current surveillance practice standards require the collection of random 4-quadrant biopsy specimens over every 1 to 2 cm of BE (Seattle protocol) to detect dysplasia with the assistance of white light endoscopy, in addition to performing targeted biopsies of recognizable lesions. This approach is labor-intensive but should currently be considered state of the art. Chromoendoscopy, virtual chromoendoscopy (e.g., narrow band imaging), and confocal laser endomicroscopy, in addition to high-definition standard endoscopy, might increase the diagnostic yield for the detection of dysplastic lesions. Until these modalities have been demonstrated to enhance efficiency or cost effectiveness, the standard protocol will remain careful examination using conventional off the shelf high-resolution endoscopes, combined with as longer inspection time which is associated with increased detection of dysplasia.
INTRODUCTION
In the Western world, esophageal adenocarcinoma (EAC) is among the cancers with the most rapidly increasing incidence rates and lowest survival rates of any cancer type.1,2 Barrett esophagus (BE), a well-established premalignant condition associated with EAC, is characterized by the metaplastic transformation of squamous epithelium to a columnar-type epithelium that contains goblet cells (intestinal metaplasia), which is evident upon histological evaluation. Progression to adenocarcinoma is believed to occur through a sequence of changes involving nondysplastic BE (NDBE), low-grade dysplasia (LGD), and high-grade dysplasia (HGD) before the final progression to EAC.3-8
The aim of endoscopic surveillance of BE is to detect dysplasia (HGD and intramucosal cancer [IMC]), which can subsequently be treated using endoscopy and cured before the progression to invasive cancer.9-11 These high-risk lesions are often subtle and difficult to detect using standard white light endoscopy (WLE). In a recent meta-analysis on resection-based studies, occult cancer was found in an average of 39.9% of patients in whom esophagectomy had been performed for BE with HGD.8 Current surveillance practice standards require the collection of random 4-quadrant biopsy specimens over every 1 to 2 cm of the BE (Seattle protocol) in order to detect dysplasia with the assistance of WLE. This protocol has been adopted as the standard of care despite many limitations, including the extratime and cost to acquire and interpret the many biopsies, which consequently leads to poor adherence by physicians.12,13 However, advanced imaging technologies can increase the diagnostic yield for the detection of BE dysplasia or cancer.14-17 A recent meta-analysis and systematic review18 based on 14 studies about advanced imaging technologies, including chromoendoscopy (CE) and virtual chromoendoscopy (VC), found that the use of these technologies increased the diagnostic yield for the detection of dysplasia or cancer by 34% (95% confidence interval, 20 to 56; p<0.0001) versus the current standard of care (WLE/random biopsy specimen). This ability to detect subtle mucosal abnormalities that harbor HGD/IMC might enable endoscopists skilled in the assessment of BE to perform targeted rather than random biopsies. The following review summarizes the most studied advanced imaging technologies available, as well as those in development, for the diagnosis of premalignant lesions (HGD/IMC) in BE.
ENDOSCOPIC DIAGNOSIS OF BARRETT ESOPHAGUS: PRAGUE CIRCUMFERENCE AND MAXIMUM LENGTH CLASSIFICATION
The diagnosis of BE should be suspected at endoscopy and confirmed with histology.19 Endoscopic suspicion of BE appears as an abnormal salmon-colored mucosa in the lower esophagus, above the tops of the gastric folds, which replaces the normal pale pink-colored esophageal mucosa. At this stage, without diagnostic histology, the endoscopy report should include the concept endoscopically suspicious for esophageal metaplasia (ESEM), especially in suspected short segments, and biopsies should be collected to confirm the presence of intestinal metaplasia (IM). However, despite recent advances in endoscopic imaging techniques, the diagnosis of ESEM does not require any methods other than conventional WLE. Multiple random biopsies should be collected in accordance with the Seattle protocol because histological confirmation of IM is an essential requisite for the diagnosis of BE, as well as a tool for the stratification of patients with NDBE, LGD, or HGD to ensure further surveillance and treatment.20
Traditionally, BE was arbitrarily classified as a short-segment disease (<3 cm) or long-segment disease (≥3 cm) according to the length of the ESEM. However, it is not clear whether this classification is clinically meaningful or affects management. The extent of BE on endoscopic examination can also be measured according to the Prague classification circumference and maximum length (C&M) criteria.21 This method has been validated and shown to provide high interobserver agreement.22 The Prague C&M criteria should be used to ensure optimal communication among physicians and clinical research homogeneity.
ENDOSCOPIC DETECTION OF DYSPLASIA AND CANCER
The endoscopic detection of dysplasia within BE is problematic even for skilled endoscopists. Several endoscopic platforms have been developed to improve the endoscopic detection of dysplasia in cases of BE. These platforms aim to minimize random sampling and facilitate targeted endoscopic resection in patients with confirmed HGD or IMC. In addition, it is hoped that the platforms will improve assessment of the disease extent and minimize the risk of missed synchronous lesions. Visible lesions suspicious for dysplasia should be targeted separately for biopsy and should be described precisely with regard to their location, size, and macroscopic aspect, according to the Paris classification.23 The macroscopic aspects of the lesion can be used to predict the risk of submucosal invasion and therefore determine whether endoscopic or surgical treatment will be required. While nodular or depressed ulcerated lesions are easy to recognize, the flat and occult lesions are an endoscopic challenge.
HIGH-DEFINITION ENDOSCOPY
Over the last 3 decades, flexible endoscopes have evolved from fiber-optic devices to the most recent HD endoscopes and associated monitors. These HD endoscopes contain charge-coupled chips with >1,000,000 pixels. These latest innovations have exponentially increased our ability to inspect and visualize subtle mucosal details. Despite significant improvements in image quality, the fundamentals of good endoscopy, particularly careful and thorough inspection by an educated eye, remain the most important tools for dysplasia detection. The adage of looking but not seeing might well account for some of the subtle lesions being missed by endoscopists who are pressurized by the time constraints of clinical practice24 and their lack of skills to recognize occult lesions. The importance of a longer inspection time was highlighted in a recent post hoc analysis of a trial that evaluated BE surveillance, in which it was reported that endoscopically suspicious lesions were more likely to be identified and patients more likely to receive a diagnosis of HGD or IMC when they were inspected for a longer time.25
CHROMOENDOSCOPY
CE is defined as the topical application of contrast agents to mucosal surfaces within the gastrointestinal (GI) tract to enhance visualization of mucosal details and it has been extensively studied. A variety of agents have been clinically utilized, and they can be categorized as absorptive (methylene blue [MB], toluidine blue, Lugol iodine), reactive (Congo red, phenol red), or contrast (indigo carmine [IC]). Generally, these agents are delivered to the target mucosa using a dedicated spray catheter. Mucosal inspection is usually performed using WLE, but additional modalities such as magnification endoscopy, optical image enhancement (e.g., narrow band imaging [NBI]), and confocal endomicroscopy can be used to evaluate suspicious abnormalities. While CE has been described as an advanced imaging technique, the use of stains is distinctly low-tech, as most of the dyes are inexpensive and widely available.24
Historically, MB was the first agent to be effective for the detection of HGD/IMC in BE, as shown by Canto et al.26 MB is an absorptive stain that is actively absorbed by the intestinal and colonic mucosa. The use of MB in the esophagus has been studied extensively, given its ability to positively stain the IM characteristic of BE while sparing the normal gastric and squamous esophageal mucosa. While the existing evidence is conflicting, several of the largest studies have suggested that MB enhances the detection of IM with fewer biopsies, compared with traditional surveillance schedules.27-29 However, a recent meta-analysis of nine studies concluded that MB CE did not offer an advantage over random biopsies for the detection of IM and dysplasia.30 Further limiting the potential use of MB in the esophagus is the somewhat laborious application process, which involves prespraying of the mucosa with a mucolytic agent and irrigating it extensively following the stain application, thus increasing the risk of aspiration. In contrast to MB, IC is not absorbed by the mucosa because it is not a vital stain. IC is simple and easily used, and has demonstrated its utility in BE surveillance when combined with magnification endoscopy.31 Further studies are needed to determine the efficacy of IC in clinical practice.
Acetic acid (AA) is a widely available, inexpensive, easy to used weak acid that facilitates mucosal contrast enhancement when applied to the surface epithelium; therefore, it has been used with both conventional WLE and magnification endoscopy to detect BE and associated dysplasia. Conflicting studies regarding its utility have been published.32-35 Balsamic vinegar, an agent that combines the advantages of CE with the structural enhancement of AA, has also been studied with regard to its use in the esophagus in a recent feasibility study that found accuracy, sensitivity, and specificity rates for BE detection as 90%, 100%, and 82%, respectively.36
One could speculate that there is a hidden, yet to be discovered vegetable or fruit extract that might prove to be the magic bullet that enhances the characteristics for the detection of occult lesions in BE.
VIRTUAL CHROMOENDOSCOPY
The evolution of digital endoscopes has facilitated the recent development of a type of digitally enhanced imaging that is analogous to traditional CE, but achieved with optical filters or the use of selective wavelengths of light. This recently developed technique is colloquially known as VC; this type of imaging is based on the principle that light penetrates tissues to variable depths based on wavelength, with blue light (shorter wavelengths) penetrating less than red light (longer wavelengths). Therefore, NBI (Olympus, Tokyo, Japan) uses blue (415 nm) and green (540 nm) light to construct endoscopic images that highlight superficial mucosal details such as capillaries and pit patterns (Fig. 1). The related but competing technologies of i-SCAN (Pentax, Tokyo, Japan) and FUJI Intelligent Chromo Endoscopy (Fujifilm, Tokyo, Japan) use the same concept and achieve similar results through the use of digital filters following image acquisition with white light. VC features several advantages over traditional CE, including widespread availability on most new endoscopes, the ability to toggle repeatedly from the normal to the enhanced image with the press of a button, and avoidance of the laborious and often nonuniform application of contrast using a spray catheter. VC has therefore gained popularity among practicing endoscopists for a range of clinical uses.24
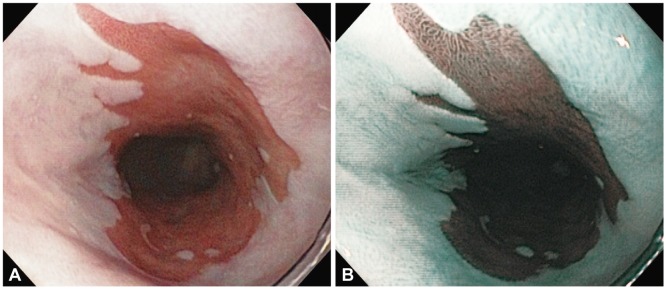
(A) White light image of Barrett esophagus C5M7. (B) Corresponding narrow band imaging Barrett esophagus image showing the regular villous architecture.
The ability of NBI to enhance the detection of BE and associated dysplasia has been studied in several prospective trials (Fig. 2).37,38 A randomized crossover trial of 123 patients found that NBI without magnification detected a higher proportion of patients with dysplasia (30% vs. 21%; p=0.01), as well as a comparable number of patients with IM but with fewer biopsies (3.6 vs. 7.6; p<0.0001).37 Similarly, a tandem study of 65 patients yielded higher rates of HGD (18% vs. 0%) and LGD (57% vs. 43%) with fewer collected biopsies (8.5 per patient vs. 4.7 per patient; p<0.01).38 A meta-analysis of eight studies involving 446 patients compared the NBI-based diagnosis (with magnification) of HGD and specialized IM (SIM) to those of histopathology, which is considered as the gold standard. The study reported high rates of sensitivity for both HGD (96%) and SIM (95%), with a better specificity for HGD (94%) than for SIM (65%).39 An issue complicating the implementation of NBI and targeted biopsies into clinical practice has been the lack of uniformity with regard to classification systems for the mucosal and vascular patterns observed with NBI. Additionally, the interobserver agreement regarding NBI images of IM and dysplasia has been only moderate between both expert and nonexpert endoscopists.40 Targeted NBI-guided biopsies have therefore not replaced the use of random biopsies for routine surveillance in BE, and careful inspection using WLE remains a key factor for the detection of subtle lesions. A confounding issue regarding the use of both chemical CE and VC is the characteristic appearance of Barrett mucosa, which is often associated with inflammation.
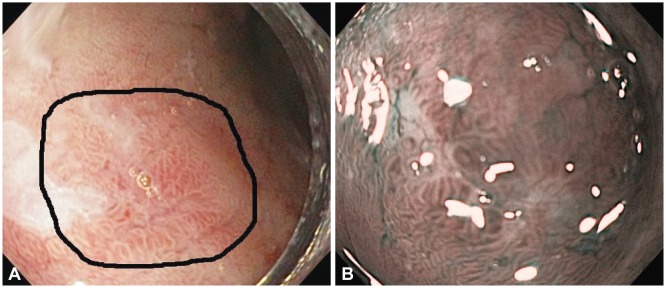
(A) White light image of slightly raised irregular mucosa. (B) Corresponding narrow band image showing a disruptive pit pattern; histology indicated intramucosal cancer involving the lamina propria (M1).
Therefore, given the results of a recent meta-analysis18 that was mentioned in the introduction, a potential recommendation for improving the diagnostic yield in dysplasia detection is to combine careful observation with VC for targeted biopsies in combination with random biopsies, which appear to have an additive effect on dysplasia detection.
CONFOCAL ENDOMICROSCOPY
Confocal laser endomicroscopy (CLE) is a developing technology that enables the high-resolution in vivo imaging of tissue microstructures at or near the level of histopathology without requiring tissue excision; this is similar to obtaining an optical biopsy. CLE, which was adapted from light microscopy, uses depth-specific tissue illumination and pinhole-limited detection to create an image from the fluorescent light reflected back from a very thin focal plane. Tissue fluorescence is achieved using intravenously or topically applied contrast agents, with intravenous fluorescein being the most common. There are currently two commercially available devices: an endoscope-based system (eCLE) that is fully integrated into the tip of a conventional endoscope (Optiscan Pty., Ltd., Notting Hill, Australia; Pentax), and a probe-based system (pCLE) that can be passed down the working channels of a range of standard endoscopes (Cellvizio; Mauna Kea Technologies, Paris, France). The potential of CLE to enhance the detection of dysplasia, while decreasing the number of required biopsies, is a concept that has generated significant academic interest.24
An initial report of 63 patients with BE found that CLE could predict BE and associated neoplasia with a sensitivity of 98.1% and 92.9%, and a specificity of 94.1% and 98.4%, respectively.15 The interobserver agreement was high (κ=0.843). To date, multiple studies have evaluated CLE with promising results.41-45 A multicenter study of 101 patients found that the addition of pCLE to HD-WLE significantly improved the detection of neoplasia;41 the reported sensitivity and specificity of HD-WLE were 34.2% and 92.7%, respectively, compared with 68.3% and 87.8% for combined pCLE and HD-WLE (p=0.002 and p<0.001). Although several preliminary studies were performed in patients referred with suspected HGD or neoplasia, a recent study in an unenriched population undergoing surveillance for nondysplastic BE found that the use of pCLE in addition to WLE enhanced the detection of dysplasia (28%), compared with that of WLE alone (10%; p=0.04).42 The promising results with this probe-based system were recently replicated by Canto et al.43 using an endoscope-based platform. They demonstrated that the combination of WLE and eCLE resulted in a 4-fold increase in the diagnostic yield of BE neoplasia, compared with that of WLE alone.43 Less impressive were the results from a trial of 68 patients across three centers in which the performance of pCLE was assessed against WLE. That trial found that while the specificity and negative predictive value of pCLE for excluding neoplasia were high (95% and 92%, respectively), the sensitivity and positive predictive value were both low (12% and 18%, respectively).44 The use of CLE for the evaluation of residual metaplasia after the ablation or resection of BE has also been assessed. One hundred nineteen patients were evaluated using HD-WLE and CLE, with no difference in the number of optimally treated patients between the two groups.45 The ongoing refinement of the technical aspects of CLE was demonstrated in a study by Gorospe et al.,46 who used a new bioprobe to evaluate ex vivo specimens and reported improved accuracy with a novel fluorescence intensity criterion.
Despite the theoretical advantage of a technique that offers optical biopsy, the practical issue is that this technology has never been adopted by most endoscopists. These devices are expensive, have long learning curves, and are confounded by the limitation of the tiny field of view. These practical issues are supported by the fact that these devices are struggling and do not seem to be commercially successful. Perhaps in the future, the devices could be incorporated into a new platform that can investigate larger surface areas.
ENDOSCOPIC ULTRASOUND
Endoscopic ultrasound (EUS) is the most accurate tool for TNM staging of esophageal neoplasms. However, the utility of EUS for the staging of early Barrett neoplasia (HGD/IMC) prior to endoscopic or surgical treatment remains debatable. In superficial Barrett neoplasia, there is evidence that EUS may both overstage and understage invasion in a significant proportion of cases when used as a single modality. Even with high-frequency probes, it is difficult to distinguish HGD from IMC or cancers that have invaded the submucosa.47-52 In cases of known cancer or suspected advanced pathology, EUS remains a useful method for assessing lymph node metastasis. However, it has limited value in the pretherapeutic algorithms of patients with early Barrett neoplasia.53 Endoscopic mucosal resection of suspected superficial Barrett neoplasia should be performed for accurate diagnostic staging and therapy.
ENDOCYTOSCOPY
Endocytoscopy (EC) involves high-level magnification endoscopy (up to ×1,400) that permits a real-time microscopic inspection of the mucosa. Unlike confocal laser microscopy, EC uses optical lenses alone to achieve the required magnification and is therefore limited to visualization of the superficial mucosa. While it is not commercially available outside Japan, both probe and endoscope-based systems have been investigated in other countries. Mucosal staining is required and has generally been achieved with topical MB and crystal violet. A study that evaluated patients with BE found that an adequate assessment of EC images was impossible in 49% of sites at ×450 magnification and in 22% of sites at ×1,125 magnification.54 The results of this study helped to conclude that currently, endoscopic histology using EC lacks sufficient image quality to assist with the identification of neoplastic areas when not supported by macroscopic evidence. This device is being researched and might have a role in the near future.
AUTOFLUORESCENCE
The use of autofluorescence (AF) during endoscopy is based on the principle that the mucosa contains variable amounts of fluorophores (biological substances that emit fluorescent light when exposed to light of a shorter wavelength) and that the different fluorescent signatures or patterns could be used to discern the normal mucosa from dysplasia. This is a wide-field CE-analogous imaging technique that has been evaluated in surveillance scenarios primarily in the esophagus, stomach, and colon. An initial study evaluating AF in 60 patients with BE found that AF increased the detection of HGD/EC, compared with WLE, but was associated with a high false positive rate55 because of a high level of confounding mucosal inflammation. In subsequent studies, an endoscopic trimodal platform combined AF, HD-WLE, and NBI in an effort to improve specificity. In a prospective multicenter study that assessed this trimodal approach, the use of WLE alone helped in the identification of only 59% of the 27 patients with neoplasias that were identified using AF. The use of NBI in addition to AF reduced the false positive rate from 81% to 26%.56 Two further studies evaluated trimodal imaging in high and intermediate-risk populations and concluded that this technique did not significantly increase the rate of dysplasia diagnosis when compared with that of WLE with random biopsies.57,58
OPTICAL COHERENCE TOMOGRAPHY
Optical coherence tomography (OCT) is a novel technique that relies on light backscattering to obtain both cross-sectional and 3-dimensional (3D) images of tissue microstructures. These images are visually analogous to viewing a coarse black and white histological specimen. OCT uses reflected light to construct an image, similar to the use of acoustic waves in ultrasound. To date, GI tract scanning has been achieved by inserting a probe through the working channel of a regular endoscope. While neither a water interface nor tissue apposition is required, the depth of scanning achieved is generally limited to 1 to 2 mm due to light scattering by tissues. A study that assessed the presence of dysplasia in BE used 177 biopsy-correlated images to evaluate a novel dysplasia index, yielding sensitivity and specificity rates for HGD/EC of 83% and 75%, respectively.59 OCT might also prove useful for assessing sub-squamous residual BE after ablation. Tsai et al.60 evaluated 33 patients with 3D OCT both preradiofrequency and postradiofrequency ablation (post-RFA) and found that the thickness of BE correlated with the likelihood of complete eradication and that the presence of persistent glands immediately following RFA predicted residual BE at follow-up.60 Although the 3D reconstruction capability is exciting, a current limitation of this technology is its inability to differentiate between the presence of dysplastic and nondysplastic glands in subepithelial BE. If the resolution and interrogation depth could be improved, OCT would have a major impact on the follow-up of mucosal dysplasia after ablation.
MOLECULAR IMAGING
Molecular imaging can be described as inclusive of modalities that enable the visualization of disease-specific morphologic, functional, cellular, and molecular changes in tissues based on differences in the specific molecular signatures of cells or whole tissues beyond differences in glandular morphology, nuclear morphology, or vascular alterations associated with neoplasia. Lesion identification and characterization based on molecular changes, rather than alterations in morphology or topography has the inherent potential to increase the efficacies of endoscopic surveillance and screening programs.24 In 2012, Bird-Lieberman et al.61 reported the use of a fluorescently conjugated wheat germ agglutinin (a lectin) for the endoscopic visualization of high-grade dysplastic lesions in patients with BE, which were not detectable by conventional endoscopy with a high signal to background ratio of >5. Molecular imaging methods could revolutionize the detection of dysplasia when combined with an appropriate endoscopic imaging device that provides a wide field of view and highlights abnormalities in real time with a high level of accuracy. Although the latest step in the journey of dysplasia detection has been taken, the field is hindered by the absence of safe, reliable, and inexpensive biomarkers.
CONCLUSIONS
The development of endoscopic imaging, from the use of early fiber-optic prototypes to the currently available high-definition instruments, has dramatically changed the paradigms of dysplasia and cancer detection in BE. Advanced imaging modalities, particularly CE and VC, appear to offer significant increase in the diagnostic yield with regard to dysplasia/cancer detection among patients with BE. In particular, VC seems to provide a more consistent and robust effect. The availability of VC is universal, given the current equipment. An additional advantage is that VC does not require the application of contrast agents, which might be time consuming and adds extra expense to the procedures.
A recent meta-analysis18 suggested that VC might be the current technology of choice for the surveillance of patients with BE in order to improve the diagnostic yields in targeted dysplasia detection when combined with random biopsies.
Despite the scientific and technological advances discussed in this review, the detection of dysplasia continues to depend upon good endoscopic techniques, including careful and timed examinations by an educated eye and the use of conventional off the shelf high-definition endoscopes. The endoscopic detection of occult lesions in BE remains a clinical challenge and an opportunity for future technological developments. The future might involve a multimodal endoscopic platform that includes the advantages of improved detection and simultaneous staging of mucosal disease.
Notes
The authors have no financial conflicts of interest.