Is Radical Surgery Necessary for All Patients Diagnosed as Having Non-Curative Endoscopic Submucosal Dissection?
Article information
Abstract
If a lesion does not meet the expanded indication criteria for treatment with endoscopic therapy for early gastric cancer or has a positive resection margin, it is regarded as suitable for non-curative resection. Non-curative resection is closely related to the risk of local recurrence, lymph node metastasis, and poor prognosis. If the result is confirmed as non-curative resection, additional treatment should be considered depending on the risks of residual tumor, local recurrence, and lymph node metastasis. As lymphatic invasion is the most important risk factor of recurrence and poor prognosis, surgical treatment should be considered if lymphatic invasion is present. If patients are not suitable for additional surgery owing to old age or coexisting severe disease, close surveillance can be an alternative treatment option.
INTRODUCTION
Gastric cancer is the fourth most common cancer in the world and has the second highest cancer-related mortality among all cancer types [1]. Early detection and treatment of gastric cancer are known to significantly reduce its associated mortality [2]. The diagnostic rate of early gastric cancer (EGCa) is increasing owing to the increase in the number of individuals who undergo health screening and the expansion of national cancer screening programs. Therefore, the importance of EGCa treatment strategy is increasing.
Traditionally, the standard therapy for gastric cancer is surgery. In recent years, endoscopic treatment such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been used for the treatment of EGCa, and clinical results have been reported to be as good as those of surgery [3,4]. The outcome of endoscopic resection for EGCa has been reported to be inferior to that of surgery. Thus, endoscopic resection is accepted as the standard treatment for EGCa with no or very low risk of lymph node metastasis [5,6].
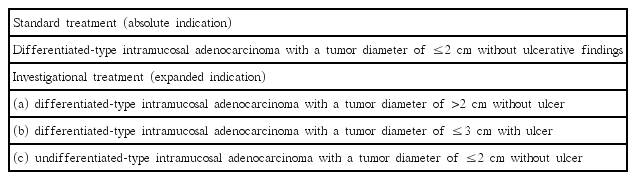
Endoscopic treatment of EGCa should enable curative resection in the absence of lymph node or distant metastasis. In the past, the absolute indication for endoscopic treatment of EGCa was differentiated intramucosal cancer measuring <2 cm for the elevated type or 1 cm for the depressed type without ulceration [7]. However, the expansion of the indications for the endoscopic treatment of EGCa has become an important issue because ESD is widely used as the standard treatment for EGCa. As the number of elderly patients who cannot tolerate operation is increasing and quality of life has become an important element of therapy, these factors have become an important background for the expansion of treatment indications.
The indication for endoscopic therapy has expanded because many articles reported that several patients with EGCa have very low risk of lymph node and distant metastases [8]. The expanded indication was introduced to include tumors clinically diagnosed as (a) differentiated-type intramucosal adenocarcinoma with a tumor diameter of >2 cm without ulcer or (b) ≤3 cm with ulcer, and (c) undifferentiated-type intramucosal adenocarcinoma with a tumor diameter of ≤2 cm without ulcer (Table 1) [9,10].
Endoscopic resection of EGCa can be performed in accordance with the absolute and expanded indications defined on the basis of tumor differentiation type, size, ulcer status, and depth of invasion. However, endoscopic curative resection of EGCa is not always possible, and the use of non-curative resection with a risk of lymph node metastasis is increasing according to the expansion of the treatment indication. Gastrectomy with lymph node dissection has become the standard management in non-curative resection owing to the risk of local recurrence and lymph node metastasis [11,12]. As the average life expectancy and the elderly population or number of patients with severe coexisting diseases increase, the number of cases of redo-ESD or argon plasma coagulation, or simple follow-up has been increasing in non-curative endoscopic resection.
The purpose of this paper was to discuss the strategies for additional treatment after endoscopic non-curative resection of EGCa.
DEFINITION OF NON-CURATIVE RESECTION
Physicians should consider two factors for curability evaluation in the endoscopic treatment of EGCa, namely completeness of the primary tumor control by endoscopy and the possibility of lymph node metastasis. Curative resection is defined as one-piece resection (en bloc) of tissue with no evidence of tumor cells in the lateral or vertical resection margin under microscopic examination and no evidence of vessel and lymphatic invasion. In the Japanese gastric cancer treatment guideline in 2014, absolute and expanded curative resection criteria are presented according to the characteristics of the removed tumor (Table 2) [9]. If the lesion does not meet these curative criteria, it is indicated for non-curative resection.
FREQUENCY AND RISK FACTORS OF NON-CURATIVE RESECTION
ESD is widely used for EGCa therapy, and the non-curative resection rate in ESD cases is lower than that in EMR cases. On the basis of a Japanese study that compared the non-curative resection rate between EMR and ESD, the non-curative resection rate was 36.7% in the EMR group in the 1990s and 17.0% in the ESD group in the 2000s.
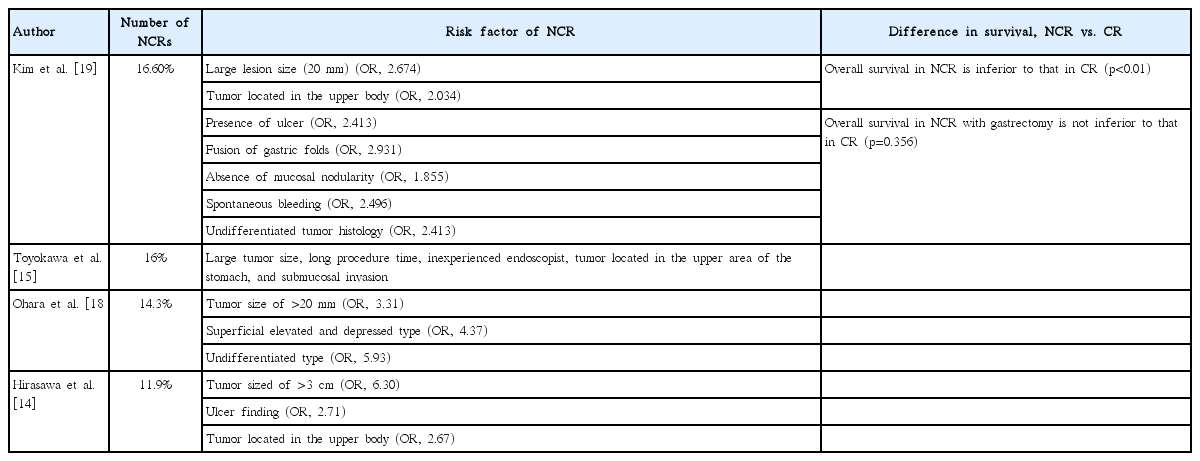
The incidence of non-curative resection after endoscopic resection of EGCa has been reported to be approximately 11.9%–18.5% according to various reports (Table 3) [13-17]. Various studies have analyzed the risk factors of non-curative endoscopic resection of EGCa (Table 3).
In a Japanese study on ESD of EGCa, the risk factor of non-curative resection had been reported to be a lesion size of >3 cm, presence of ulceration, and tumor located in the upper body [14]. Another Japanese study reported that tumor size of >20 mm, the superficial elevated and depressed types, and the undifferentiated type increase the risk of non-curative resection [18]. According to a large retrospective Korean study, large tumor size (≥2 cm), tumor located in the upper body, the presence of ulcer, fusion of gastric folds, the absence of mucosal nodularity, spontaneous bleeding, and undifferentiated tumor were the risk factors of non-curative resection [19]. As mentioned earlier, the risk tended to increase with larger tumor sizes, tumors located in the upper area, and undifferentiated tumors. In addition to the characteristics of the lesion at the time of the procedure, a study compared endoscopic techniques and the risk factors of non-curative resection, and histological discrepancy before and after endoscopic treatment [15]. In the previous study, the causes of non-curative resection were classified into three categories as follows: inadequate technique, preprocedural misdiagnosis, and problems in histological diagnosis. Large lesion size, long procedure time, and inexperience of the endoscopist were the risk factors of inadequate technique. The upper area of the stomach and cancer with submucosal invasion were associated with a higher risk of non-curative resection due to preprocedural misdiagnosis and problems in the histological diagnosis. Various techniques such as endoscopic ultrasonography (EUS), magnifying endoscopy with narrow-band imaging, computed tomography, and positron emission tomography have been introduced to lower preprocedural misdiagnosis. However, in one Korean study, EUS and computed tomography imaging equipment showed 80%–90% accuracy in predicting lesion invasion and lymph node metastasis, but the use of these imaging techniques has been reported to have limitations [20]. In a meta-analysis of whether EUS is useful for assessing the staging depth of invasion of EGCa, EUS was reported to be ineffective for accurately predicting depth of invasion before EGCa treatment [21]. The estimation of the extents of lesion invasion and lymph node metastasis using imaging studies is still limited, and further research is needed.
In summary, the risk factors of non-curative resection with ESD for EGCa are tumor size (>2–3 cm), tumor located in the upper body, undifferentiated cell type, inadequate endoscopic technique and preprocedural misdiagnosis, and discrepancy of histology before and after ESD.
FREQUENCY AND RISK FACTORS OF POSITIVE HORIZONTAL MARGIN
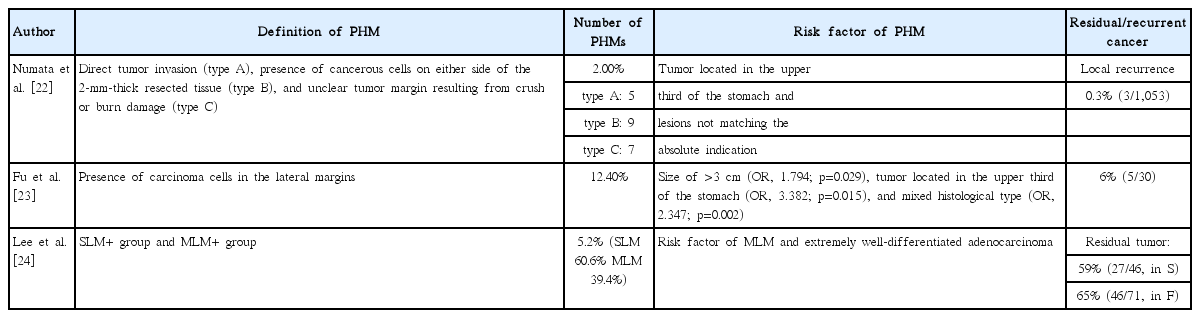
Among cases of non-curative resection, only those with a positive horizontal margin were reported to have superior prognosis as compared with other cases of non-curative resection [9]. Therefore, careful consideration should be given to the treatment strategy in terms only of a positive horizontal margin. According to previous reports, the positive horizontal margin after ESD was reported to be 2%–12.4% (Table 4). Several studies reported the risk factors of positive horizontal margin after ESD in EGCa. According to the study by Numata et al., the risk factors of positive horizontal margin in ESD of EGCa were tumor located in the upper third of the stomach and non-matching the absolute indication for endoscopic treatment of EGCa [22]. The percentage of the positive horizontal margin after ESD was 2%, and local recurrence was found only in 3 patients and managed with redo ESD.
Another Japanese study reported that the risk factors of positive horizontal margin were tumor size of >3 cm (odds ratio [OR], 1.794; p=0.029), tumor located in the upper third of the stomach (OR, 3.382; p=0.015), and mixed histological type (OR, 2.347; p=0.002) [23]. In a Korean study of 1358 ESD-treated patients with EGCa that aimed to identify the risk factors of positive horizontal margin, the authors concluded that extremely well-differentiated adenocarcinoma and multiple lateral margins were the only risk factors [24].
RISK OF LOCAL RECURRENCE AND LYMPH NODE METASTASIS AFTER NONCURATIVE RESECTION
According to a prospective and retrospective study that performed ESD of EGCa, incomplete resection or piecemeal resection is closely related to local recurrence of gastric cancer [25,26]. In case of non-curative resection after ESD of EGCa, additional treatment should be performed after identifying the risks of residual tumor and recurrence.
According to various reports, the incidence rate of residual tumor was 24.6%–34.9% in the analysis of specimens obtained after surgical treatment with non-curative resection of EGCa [27,28]. On the basis of studies about the risk factors of residual tumors, larger tumor size (2–3 cm) [27,29], positive horizontal margin [27,29,30], positive vertical margin [29], presence of lymphovascular invasion [29,30], diffuse type of cancer [30], and piecemeal resection [30] were the risk factors of residual tumor.
Many reports have identified the risk factors of local recurrence after non-curative resection. According to the Korean study conducted by Kim et al. to identify the risk factors of local recurrence in positive horizontal margin, tumor size of >2 cm (OR, 4.48), positive horizontal margin (+), and resection length of >6 mm (OR, 7.65) were the risk factors of local recurrence [31]. On the basis of the studies about the risk factors of recurrent tumors after endoscopic resection, presence of lymphovascular invasion, long segment of the positive horizontal/vertical margin, and inclusion from the expanded indications were the risk factors of residual tumor [31,32]. In a study in patients with EGCa who underwent a non-curative resection with a positive vertical resection margin, the incidence of residual tumor/local recurrence was 33.3% and the total length of lateral resection was a risk factor of residual tumor/local recurrence [33]. In summary, the factors associated with residual tumor and local recurrence after endoscopic resection of EGCa were as follows: piecemeal resection, tumor size of >2–3 cm, submucosa invasion, and lymphatic and vascular invasions.
The information about the prevalence and risk factors of lymph node metastasis with non-curative resection after endoscopic treatment of EGCa is greatly helpful for future treatment decisions. However, the criteria for tumors that affect lymph node metastasis of EGCa are controversial. According to a study by Gotoda et al., no lymph node metastasis was found in undifferentiated intramucosal carcinomas of <2 cm without ulcer and submucosal invasive carcinomas of <3 cm without lymphovascular invasion [34]. This was the background of the expansion of the indications from the absolute indications of endoscopic resection of EGCa. However, in some reports, lymph node metastasis was observed in undifferentiated intramucosal tumors of <2 cm or differentiated submucosal tumors of <3 cm in diameter [35-38]. A meta-analysis of lymph node metastasis of undifferentiated EGCa revealed that undifferentiated carcinoma of >2 cm in size, SM invasion, presence of lymphovascular invasion, presence of an ulcer finding, and histological type (non-signet ring carcinoma) were more related to lymph node metastasis [39]. Moreover, in a meta-analysis that compared the incidence of lymph node metastasis between the absolute and expanded indications according to Japanese guidelines, Abdelfatah et al. showed a high risk of lymph node metastasis in undifferentiated tumors of <2 cm or submucosal tumors of <3 cm in diameter [40].
According to various reports, lymph node metastasis was observed in approximately 5.2%–9.3% of patients who underwent surgery after non-curative resection [28,41,42]. In several studies that performed gastrectomy and lymphadenectomy after non-curative endoscopic resection, lymphovascular invasion and SM2 or higher were independent risk factors of lymph node metastasis [29,41]. However, lymph node metastasis was rare in patients with only a positive horizontal margin after non-curative resection [13,43]. In a meta-analysis of the factors associated with lymph node metastasis in 1,720 patients who underwent additional gastrectomy after non-curative resection, vascular invasion, lymphatic invasion, SM2 invasion, and positive vertical margin significantly correlated with metastasis [42]. These reports suggest that the risk of lymph node metastasis is high in the presence of lymphatic invasion, vascular invasion, positive vertical margin, and SM2 and higher, but the risk is low in patients with positive horizontal margin alone. In a recent report and meta-analysis, undifferentiated or submucosal tumors of <2–3 cm in size had a risk of lymph node metastasis [39]. Thus, further research is needed in the future to clarify the size and tumor characteristics.
LONG-TERM OUTCOME AFTER NONCURATIVE RESECTION
Additional treatment with non-curative resection of EGCa should be chosen according to the risk of residual tumor, recurrence, and lymph node metastasis by assessing the tumor size, depth of invasion, degree of differentiation, positivity of lateral and vertical resection margins, and lymphatic and vascular invasions. Various early studies showed that gastrectomy with lymphadenectomy would result in better prognosis if non-curative resection has a high risk of residual or recurrent tumor and lymph node metastasis [13,44,45].
The study about the natural course of differentiated EGCa treated with non-curative resection reported overall 3- and 5-year survival rates of 82.9% and 77.1%, respectively. Underlying disease and lymphovascular invasion were the risk factors of poor prognosis. The overall 3- and 5-year survival rates without lymphovascular invasion were 86.1% and 81.8%, respectively, which were superior than those in patients with lymphovascular invasion (61.9% and 42.4%, respectively; p<0.001) [37]. They recommended that additional surgery should be considered for patients with lymphovascular invasion.
In a cohort study that compared mortality and recurrence rates among patients who initially underwent standard surgery (group A), patients who underwent additional surgery after non-curative ESD (group B), and patients who did not undergo additional surgery after non-curative ESD (group C), the overall mortality and recurrence rates were not significantly different between groups A and B [46]. However, the mortality and recurrence rates in group C were higher than those in group A. Thus, they concluded that additional surgery should be chosen after non-curative ESD to obtain a good prognosis. The disadvantages of these studies, however, are that the group not treated with additional surgery after non-curative ESD had significantly higher age and incidence of mortality due to associated diseases. Moreover, it is reasonable that patients with a positive horizontal margin alone and a relatively good prognosis should be excluded in the study.
Recently, several studies were conducted to compare the survival rates between patients who received additional surgery and those who underwent simple follow-up after non-curative resection except for those with a simple positive horizontal margin (Table 5). In those studies, a significant difference in overall survival (OS) but not disease-specific survival (DSS) was found between the two groups [36,41,45,47-49]. In a larger-scale Japanese retrospective multicenter study, the 3- and 5-year OS rates in the additional surgery group were higher than those in the simple follow-up group. However, the 3- and 5-year DSS rates in the additional surgery group were not significantly different from those in the simple follow-up group. Lymphatic invasion was found as an independent risk factor of recurrence in the follow-up group. They recommended additional surgery in case of lymphatic invasion after non-curative ESD. The reason for the lower OS rate in the follow-up group than in the additional surgery group is presumed to be the higher rate of coexistent disease or larger proportion of elderly patients in the follow-up group. That is, not all non-curative resections require surgery. However, if a risk of lymph node metastasis or recurrence is present, surgical treatment is indicated. In many previous reports, lymphovascular invasion has been reported to increase the risk of lymph node metastasis and recurrence. Therefore, we think that surgical treatment should be considered when lymphovascular invasion is present.
TREATMENT OPTIONS OTHER THAN SURGERY
The most commonly adopted method other than surgery is additional endoscopic treatment such redo ESD or argon plasma coagulation. Additional ESD can assess curative resection after retreatment, can preserve stomach function, does not affect quality of life, and can be performed relatively easy in elderly patients or patients with underlying diseases. However, because of the fibrosis of the submucosal layer associated with the previous procedure, the procedure is technically difficult, which may lead to increased complications (bleeding or perforation). Compared with ESD, the argon plasma coagulation method can be used safely but cannot be used to evaluate curative resection histologically after treatment. In a study of the efficacy and procedure time in secondary ESD, the procedure time in secondary ESD was longer than that in primary ESD, but no significant difference in complication was found between secondary and primary ESD [50]. They suggest that secondary ESD is safe and effective in preventing local recurrence after non-curative endoscopic resection. Other follow-up studies also described secondary ESD as a safe and effective method and recommended performing secondary ESD as soon as possible after primary endoscopic treatment [51,52].
Recently, new surgical options such laparoscopic lymph node dissection without gastrectomy and sentinel node navigation surgery have been introduced if the risk of recurrence is expected to be low [53,54]. If patients desire to preserve stomach function to increase quality of life, new surgical options may be considered as alternative treatments. However, further research on stability is needed.
TREATMENT STRATEGY OF NONCURATIVE RESECTION
If non-curative resection is performed after endoscopic treatment of EGCa, further treatment decisions should be made after assessment of the risks of recurrence of stomach cancer and lymph node metastasis. In case of no or very low risk of recurrence, additional endoscopic treatment or simple follow-up without additional surgery may be used as an alternative treatment [9]. However, surgical treatment may be necessary if there is a risk of recurrence and if the patient is healthy enough to tolerate surgery.
Fig. 1 shows the modified treatment strategy of non-curative resection after endoscopic therapy for EGCa [9,55].

Modified treatment strategy after non-curative endoscopic resection of early gastric cancer [9,55]. ESD, endoscopic submucosal dissection; LVI, lymphovascular invasion; ET, endoscopic treatment. a)Tumor size of >30 mm, positive vertical margin, venous invasion, and submucosal invasion of ≥500 mm. b)If surgery is not possible in elderly patients or patients with severe coexisting disease.
If only a positive horizontal margin is present, endoscopic treatment or follow-up can be used. In other situations, the risks of recurrence of gastric cancer and lymph node metastasis should be analyzed for appropriate additional treatment. Most studies reported that lymphatic invasion is the most important risk factor of cancer recurrence and poor prognosis. In addition, tumor size of >3 cm, positive vertical margin, venous invasion, submucosal invasion of ≥500 mm were also identified as risk factors of cancer recurrence and poor prognosis [55]. Thus, in case of the presence of lymphatic invasion or two or more other risk factors, additional surgery should be considered if possible. However, if surgery is not possible, additional endoscopic treatment or close surveillance may be an alternative option especially for elderly patients or patients with severe coexisting diseases.
CONCLUSIONS
Non-curative resection after endoscopic treatment for EGCa is related to the risk of local recurrence and lymph node metastasis. The physician must predict the possibility of non-curative resection through an accurate assessment of the lesion before treatment. If non-curative resection, additional treatment should be considered according to the risks of residual tumor, local recurrence, and lymph node metastasis. Lymphatic invasion, tumor size of >3 cm, positive vertical margin, venous invasion, and submucosal invasion of ≥500 mm were the risk factors of cancer recurrence and poor prognosis. As lymphatic invasion is the most important risk factor of poor prognosis, patients with lymphatic invasion or two of more other risk factors can be surgical candidates. In case of only a horizontal margin positive or patients with old age or a coexisting severe disease, close surveillance can be an alternative treatment option.
Notes
Conflicts of Interest:The authors have no financial conflicts of interest.