Air Embolism during Upper Endoscopy: A Case Report
Article information
Abstract
Air embolism is a rare complication of upper endoscopy and potentially causes life-threatening events. A 67-year-old man with a history of surgery of cardiac carcinoma and pancreatic neuroendocrine tumor underwent painless upper endoscopy because of tarry stools. During the procedure, air embolism developed, which caused decreased pulse oxygen saturation and delayed sedation recovery. He recovered with some weakness of the left upper limb in the intensive care unit without hyperbaric oxygen therapy. The etiology, clinical manifestations, and treatments of air embolism are discussed based on the literature reports. Although air embolism is uncommon in endoscopic examinations, the patients’ outcomes could be improved if clinicians are alert to this potential complication, and promptly start proper diagnostic and therapeutic measures.
Introduction
Air embolism is an iatrogenic clinical problem that could potentially cause catastrophic events. It could occur in different clinical specialties but rarely in gastrointestinal endoscopy [1]. Endoscopy-related air embolism mostly occurs as a complication of endoscopic retrograde cholangiopancreatography (ERCP), while being relatively rare in esophagogastroduodenoscopy (EGD), endoscopic ultrasonography (EUS), colonoscopy, or sigmoidoscopy [2]. To date, only a few cases of air embolism occurring during EGD have been reported. We present a case of cerebral air embolism that occurred during EGD and EUS.
Case report
A 67-year-old man with tarry stools was admitted for painless EGD. He had a history of surgery of cardiac carcinoma and pancreatic neuroendocrine tumor 3 years previously. Before EGD, he did not receive any therapies. After he was sedated intravenously with 1 mg midazolam and 100 mg propofol, the endoscopic examination proceeded smoothly. The gas filled in the whole examination was air, not carbon dioxide (CO2). During the procedure, an anastomotic stoma was found 45 cm from the incisor teeth. Congestion, edema, and erosion in the mucous membrane of the anastomotic stoma were observed. A diverticulum formed by the closed orifice of cardiac carcinoma surgery, with a size of about 1×1 cm, was seen under the anastomotic stoma (Fig. 1A). Moreover, some brown blood clots were found inside the diverticulum (Fig. 1B). A protuberance with a size of about 2×2 cm was also seen 40 cm from the incisor teeth, the surface of which was smooth (Fig. 1C). Further EUS showed that the protuberance could be cystic (Fig. 1D). No special abnormalities were seen in the rest of EGD.

Endoscopic images. (A) The entrance of the diverticulum formed by the closed orifice of cardiac carcinoma surgery. (B) The diverticulum with brown blood clots inside. (C) A protuberance 40 cm from the incisor teeth. (D) The protuberance was proved to be cystic on endoscopic ultrasonography. (E) The diverticulum with active bleeding after flushing out the brown blood clots.
To identify whether the bleeding originated from the diverticulum, we flushed out the brown blood clots. Then, active bleeding occurred inside the diverticulum (Fig. 1E). As we were attempting to find the bleeding spot, a progressive decrease in pulse oxygen saturation occurred, with a minimum level of 82%. The circulation status was stable. Therefore, we had to stop the endoscopic procedure (12 min in total), and the patient was given manual ventilation with 100% oxygen. After 10 min, the pulse oxygen saturation gradually increased to >95%. However, 30 min later, the patient was still unconscious despite being administered with flumazenil. Physical examination revealed a positive right Babinski sign, right gazing in both eyes, and a Glasgow Coma Scale score of 6/15 with E2V1M3. An urgent computed tomography (CT) scan of the brain, chest, and abdomen was immediately performed, which showed evidence of multiple air emboli in the left and right frontal and parietal lobes of the brain (Fig. 2A). Furthermore, there were diffused gas density shadows mainly at the edge of the liver (Fig. 2B).
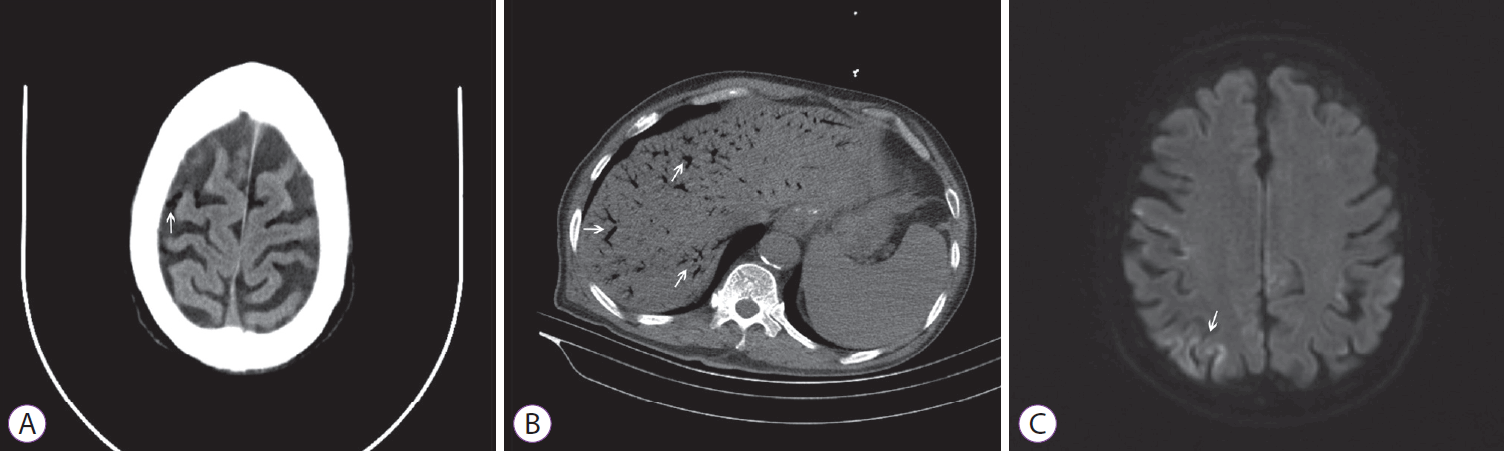
Imaging examinations. (A) Computed tomography (CT) scan showing sporadic multiple air emboli in the left and right frontal and parietal lobes of the brain (arrow). (B) CT scan showing diffused gas density shadows mainly at the edge of the liver (arrows). (C) Magnetic resonance scan showing sporadic multiple hyperintense signals in the frontal and parietal lobes in T2-weighted images (arrow).
Thereafter, the patient was transferred to the intensive care unit (ICU) for further treatment. However, owing to the restrictions of his medical conditions, he was not able to receive hyperbaric oxygen therapy (HBOT). Instead, edaravone ganglioside and citicoline were administered intravenously and an ice cap was placed on his head to help the cerebral cells recover. Iced normal saline containing norepinephrine and Yunnan white medicine was administered nasally to stop upper gastrointestinal bleeding, and omeprazole and octreotide were also administered intravenously to inhibit digestive fluid secretion. Four days later in the ICU, the patient recovered consciousness but with paralysis of the left upper limb and weakness of the right upper limb. Rehabilitation exercise was then implemented to help regain muscle strength. During the hospitalization, further cerebral magnetic resonance scanning revealed sporadic multiple hyperintense signals in the frontal and parietal lobes in T2-weighted images (Fig. 2C), whereas transthoracic echocardiography examination showed no evidence of patent foramen ovale (PFO). After nearly 1 month of therapy, the patient was discharged with grade 4 muscle strength of the left upper limb.
Discussion
Air embolism is a rare complication during gastrointestinal endoscopy, especially in upper endoscopic procedures. Mandelstam et al. [3] retrospectively estimated that 211,410 upper endoscopies were performed in 1976, and there was no episode of air embolism reported in any of the cases. Moreover, Donepudi et al. [1] searched the PubMed database and found only 41 cases of air embolism complicating various endoscopic procedures reported up to 2013. Most cases of endoscopy-related air embolism were due to ERCP operations, followed by EGD.
There are 2 conditions for the occurrence of air embolism [4]. One is a communication between an air source and the vasculature. It was considered that interruption of the mucosal barrier likely increases the risk of air embolism, including ulceration, gastric erosions, balloon dilatation, biopsy specimen retrieval, and post-surgical gastrointestinal fistula [1,5]. In our present case, we considered that the brown blood clots inside the diverticulum might be the remote hemorrhage that caused the patient’s tarry stools. Active bleeding occurred immediately after flushing out the blood clots, which could have exposed small vessels to air and provided an entry of air emboli into the portal venous system. The diffused air emboli were mainly at the edge of the liver, further indicating that the emboli probably originated from the portal venous system. The other condition is a pressure gradient favoring passage of air into the circulation. Gastrointestinal endoscopy requires air insufflation at a high rate, thus producing a pressure gradient. During gastroscopy at its full capacity, air would be infused at a rate of 30 mL/s and overpressures of up to 45 kPa could be generated in a few seconds if there is no possibility of air drainage [6]. This is considered to cause a critical air embolism if a connection with the circulation system exists [7]. Using CO2 instead of air could reduce the risk of air embolism because of the rapid diffusion and absorbability of CO2 [8]. In our procedure, the insufflation gas was air, not CO2, which might have increased the probability of gas entering the circulatory system.
After venous air embolism occurs, it could either be limited to the portal venous system or develop into a systemic embolism. The 2 most common passages in which an arterial embolism could form are through intracardiac and intrapulmonary right-to-left shunts [9,10]. Other paths including retrograde flow into cerebral veins via the superior vena cava, or air passage into the left atrium via the pulmonary veins have already been reported [11]. Among the causes of intracardiac shunts, PFO has been commonly reported. Lynch et al. [12] found that a PFO could be detected in 30% of the general population. If massive air emboli enter the pulmonary circulation, increasing the pulmonary arterial pressure and then the pressure in the right atrium, which exceeds the pressure of left atrium, the air emboli could be enforced into the systemic circulation through the PFO. In other circumstances, patients with no evidence of PFO or intracardiac defects might develop arterial embolism because the pulmonary microcirculatory filtration is overwhelmed by the rapid input and massive volume of air [13]. In our case, no PFO was found in the patient, and therefore the air emboli most likely entered the systemic system by crossing the pulmonary microcirculatory filtration.
The clinical manifestation of air embolism is determined by the amount of air entering the circulation, and whether the air is limited to the venous system or extends into the systemic circulation. In systemic air embolism, the brain is the most commonly affected organ, which could present different symptoms including consciousness alteration, hemiparesis, hemianopia, pupil asymmetry, or even comatose and death. However, patients with sedation or anesthesia would probably show delayed consciousness recovery, which might be confused with other cerebral accidents or complications of anesthesia, such as residual anesthetic agents. These complications have similar manifestations to mild cerebral arterial air embolism. In this situation, rapid diagnosis is required for timely treatment of cerebral air embolism. Most reported cases of air embolism were diagnosed with either CT or echocardiography [14]. Transthoracic echocardiography and transesophageal echocardiography have been considered to show evidence of gas bubbles in cardiac chambers. Moreover, they also assist in revealing structural abnormalities or in estimating cardiac function. Urgent CT is a highly sensitive method for the detection of gas bubbles in damaged organs during the early period. Cerebral air embolism could sometimes be distinguished from other cerebrovascular events on CT scans. However, CT scan is limited in that it could only detect bubbles over a 1.3-mm radius and might be dependent on the thickness of the CT slices [15]. Further, if gas bubbles had been absorbed, a CT scan would present no evidence of air emboli. Therefore, the absence of air does not preclude the diagnosis. Thus, CT examinations should be performed as early as possible.
The treatments of air embolism involve several maneuvers. First, the operation should be immediately stopped if possible and further embolization should be prevented. Second, highflow 100% oxygen should be administered. It has been proved that when administering 100% oxygen, the higher the partial pressure of oxygen in the circulation, the faster the clearance or reabsorption of air bubbles [16]. Third, the circulatory status should be maintained. If necessary, cardiopulmonary resuscitation should be performed without hesitation. Fourth, the patient’s position should be changed to the Trendelenburg and left lateral decubitus position, which may prevent a large air bubble from obstructing the right ventricular outflow tract. Fifth, volume expansion should be initiated. Adequate fluid therapy could decrease hemoconcentration and blood viscosity, which would benefit the microcirculation [17]. Colloid solutions are preferred over crystalloid solutions, as the latter might worsen cerebral edema [13]. Finally, the most important therapy for air embolism is HBOT. It has been reported that the mortality rate in untreated patients is >90%, whereas HBOT has reduced this rate to 7% [14]. HBOT could reduce the bubble size by increasing the ambient pressure and causing systemic hyperoxia [15]. In previous reports, HBOT showed the best prognosis within 5 h but was still beneficial up to 30 h [16,18]. HBOT has been recommended as the first-line treatment for arterial gas embolism [13].
In conclusion, air embolism could occur during endoscopic procedures and might result in catastrophic events. Prompt recognition and timely management are keys to preventing severe outcomes. HBOT is considered the best treatment for air embolism, which should be performed within 5 h.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest
