Endoscopic Self-Expandable Metal Stent Placement for Malignant Afferent Loop Obstruction After Pancreaticoduodenectomy: A Case Series and Review
Article information
Abstract
In this study, we assessed a series of our cases in which endoscopic self-expandable metal stents (SEMSs) were used to treat malignant afferent loop obstruction (ALO) that arose after pancreaticoduodenectomy (PD). We retrospectively examined the records of 7 patients who underwent endoscopic SEMS placement for malignant ALO following PD. Clinical success was achieved in all cases. The median procedure time was 30 min (range, 15–50 min). There were no cases of stent occlusion, and no procedure-related adverse events were encountered. All patients died of their primary disease, and the median overall survival period was 155 days (range, 96–374 days). A re-intervention involving endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting was performed for obstructive jaundice and acute cholangitis in 1 case. In conclusion, endoscopic SEMS placement may be an effective and safe treatment for malignant ALO that arises after PD.
INTRODUCTION
Afferent loop obstruction (ALO) caused by recurrent cancer (malignant ALO) that arises after pancreaticoduodenectomy (PD) can lead to an increase in internal pressure, which can block the outflow of bile or pancreatic juice. In addition, it often leads to the development of cholangitis or jaundice due to a loss of papillary function [1]. Conventionally, malignant ALO that arises after PD has been managed surgically. However, the general condition of patients with recurrent cancer is often not good enough to undergo surgery. Recently, endoscopic self-expandable metal stents (SEMSs) have been widely used to treat malignant gastric outlet obstruction (mGOO), whereas endoscopic SEMS placement for malignant ALO that arises after PD has only been described in case reports. Previously, we reported a case of malignant ALO arising after PD that was successfully treated by endoscopic SEMS placement [2]. However, the clinical outcomes of endoscopic SEMS placement for malignant ALO following PD remain to be evaluated. Herein, we report 7 cases of endoscopic SEMS placement for malignant ALO that arose after PD.
CASE REPORT
Patients
In this study, we retrospectively examined the records of 7 patients (3 males, 4 females; median age, 68 years; range, 56–82 years) who underwent endoscopic SEMS placement for malignant ALO that arose after PD at Kobe University Hospital between April 2010 and May 2018. The primary diseases included 5 cases of pancreatic cancer, 1 case of duodenal cancer, and 1 case of bile duct cancer. Subtotal stomach-preserving PD was performed for the primary disease in all cases. The indications for endoscopic SEMS placement included symptoms caused by cholangitis and a distended afferent loop. Six of 7 patients developed fever and elevated levels of biliary enzymes. One patient experienced severe abdominal pain due to marked distention of the afferent loop but did not develop a fever or elevated biliary enzyme levels (Table 1). The study protocol was in accordance with the Declaration of Helsinki and approved by the ethics committee of Kobe University Hospital (No. 170100).
Diagnosis of malignant afferent loop obstruction
The diagnosis of malignant ALO was determined based on the clinical presentation, laboratory data, and computed tomography (CT) findings of each case. In each case, CT scans revealed marked distention of the afferent loop due to bowel obstruction caused by a recurrent tumor (Fig. 1A). Upper gastrointestinal endoscopy was performed to confirm the diagnosis of neoplastic stenosis and manage the case.
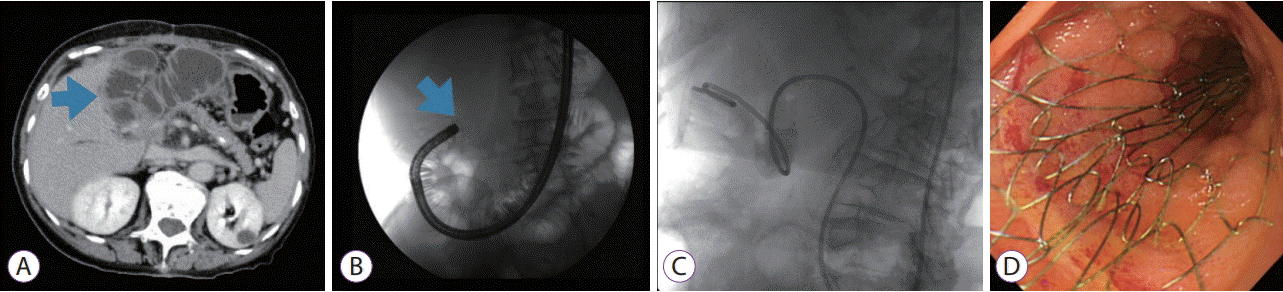
(A) A computed tomography scan revealed marked distention of the afferent loop, which was suggestive of bowel obstruction caused by a recurrent tumor. (B) An endoscope was inserted into the afferent loop. A neoplastic stenotic lesion blocked further passage of the endoscope. (C) A nasojejunal tube was placed over a guidewire to decompress the dilated afferent loop. (D) A self-expandable metal stent was inserted using the standard through-the-scope technique.
Therapeutic technique
An endoscope was inserted into the afferent loop (Fig. 1B). After reaching the neoplastic stenotic lesion, a guidewire was advanced across the stricture and into the dilated afferent loop. Then, a nasojejunal tube (N-tube) was placed over the guidewire to decompress the dilated afferent loop (Fig. 1C). After decompressing the afferent loop, the length and location of the obstruction were accurately evaluated by injecting contrast medium through the N-tube to allow safe and effective endoscopic SEMS placement. Subsequently, the endoscope was inserted into the afferent loop along the N-tube, and an SEMS was deployed across the stricture using the standard through-the-scope technique (Fig. 1D). The procedure was performed using a therapeutic gastrointestinal endoscope (GIF 1T260; Olympus Optical Co., Tokyo, Japan) with a 3.7-mm working channel, under conscious sedation. In 6 patients, Niti-S uncovered duodenal stents (Taewoong Medical, Seoul, Korea), measuring 22 mm in diameter and 80, 100, or 120 mm in length, were used. In the remaining patient, an Evolution uncovered duodenal stent (Cook Medical, Bloomington, IN, USA), measuring 22 mm in diameter and 60 mm in length, was used. The expansion of the SEMS and amelioration of the ALO were assessed on CT scans after the SEMS placement.
Definitions
Clinical success was defined as decompression of the anastomotic proximal afferent loop after SEMS placement combined with symptom relief. The procedure time was defined as the time between the insertion and removal of the endoscope. The severity of adverse events was evaluated according to the American Society for Gastrointestinal Endoscopy’s grading system [3].
Statistical analysis
Results are expressed as medians and ranges or absolute values and percentages. Overall survival was estimated using the Kaplan-Meier method. All analyses were performed using the JMP Pro software version 11.2.0 (SAS Institute, Cary, NC, USA).
Results
The results of this study are summarized in Table 2. Clinical success was achieved in all 7 cases postoperatively (100%). The median procedure time was 30 min (range, 15–50 min). There were no cases of stent occlusion. After the SEMS placement, 2 of the 7 patients received chemotherapy, while the 5 remaining patients received best supportive care. No adverse events associated with the procedure were encountered in this series. All the patients died of their primary disease, and the median overall survival period was 155 days (range, 96–374 days).
A re-intervention for obstructive jaundice and acute cholangitis was performed in 1 case (case 2). This patient presented with biliary obstruction due to tumor recurrence at a bilioenteric anastomosis at 253 days after SEMS placement. SEMS obstruction was not observed under endoscopy and fluoroscopy. The patient’s jaundice and cholangitis were treated by endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (EUS-HGAS) owing to failed biliary stenting via bilioenteric anastomosis (Fig. 2).
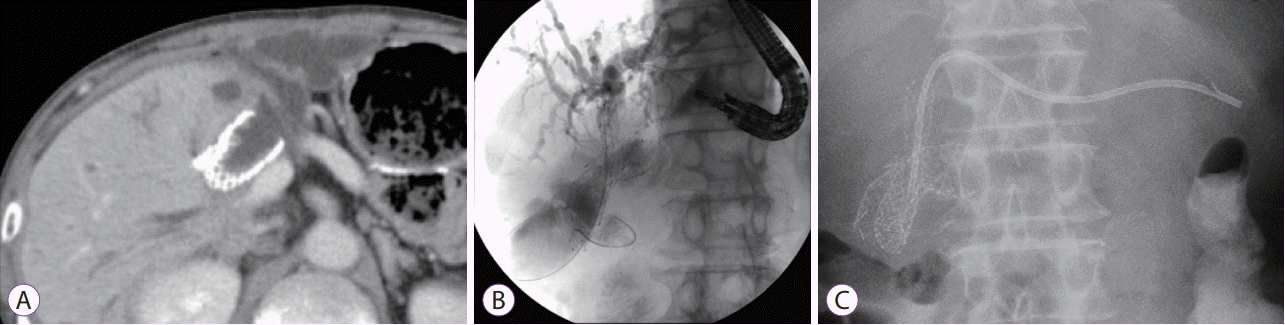
(A) Case 2 presented with obstructive jaundice due to invasive cancer involving a bilioenteric anastomosis after self-expandable metal stent (SEMS) insertion. (B) Endoscopic ultrasound-guided biliary drainage was selected. An echoendoscope was advanced into the stomach, and a 19-gauge fine needle aspiration needle was advanced into a left-sided intrahepatic bile duct (B3). Cholangiography showed stenosis of the bilioenteric anastomosis, and a guidewire was advanced through the stenotic lesion and into the afferent loop. (C) An uncovered SEMS was placed across the stenotic lesion, and a plastic stent was placed through the fistula between the gastric body and the intrahepatic bile duct.
DISCUSSION
Endoscopic SEMS placement for malignant ALO that arises after PD has only been reported sporadically in case reports [3]. Our case series is the first to focus on the clinical outcomes of endoscopic SEMS placement for malignant ALO that arises after PD.
Malignant ALO following PD can be managed via surgical or non-surgical treatment. While surgery is effective, it is also highly invasive. Many patients with recurrent pancreaticobiliary cancer are not well enough to tolerate surgery. Hence, a non-surgical treatment option is clearly preferable. Previously, malignant ALO had been treated with percutaneous transhepatic biliary drainage (PTBD). However, biliary access can be challenging in patients without jaundice or sufficiently dilated intrahepatic bile ducts. Furthermore, PTBD is known to cause severe adverse events, such as bleeding, bile peritonitis, or ascending cholangitis [4]. Endoscopic treatment approaches for ALO have emerged as feasible alternatives to surgery. Recently, endoscopic duodenal SEMS placement has been implemented as an alternative to surgical bypass for the palliation of mGOO in light of its low morbidity and mortality rates. In contrast, endoscopic SEMS placement for malignant ALO has only been reported sporadically in case reports.
During a PubMed search, we found 12 reports (23 patients) on the use of SEMS to treat malignant ALO that arises after PD (Table 3) [5-16]. Clinical success was achieved in all cases, and no adverse events were encountered. Although the data were limited, no cases of SEMS obstruction were found. In addition, none of these previous cases required re-intervention after the SEMS placement. In our case series, no procedure-related adverse events were encountered, the procedure time was not prolonged, and technical success was achieved in all 7 cases. Two of the 7 cases received chemotherapy after the SEMS placement, and both exhibited prolonged survival. There was no clinical suspicion of stent dysfunction in any patient. One patient presented with obstructive jaundice after the SEMS placement. Biliary obstruction occurred due to tumor recurrence at the bilioenteric anastomosis without SEMS obstruction. We determined that direct biliary drainage was required in this situation. However, biliary stenting via the bilioenteric anastomosis failed due to difficult cannulation of the bile duct. In this case, EUS-HGAS was performed as an alternative biliary drainage method, which led to an improvement in the patient’s condition. Furthermore, our cases were followed up until death, and no recurrent cholangitis due to obstruction of the SEMS was detected. Thus, our case series exhibited satisfactory clinical outcomes.
We speculated that the two-step approach would be useful for the safe and accurate deployment of endoscopic SEMS for malignant ALO. A N-tube was inserted into the distended afferent loop under fluoroscopy guidance for all cases, and then SEMS was deployed across the stricture after improvement of the clinical condition. This approach has 3 advantages. First, it is more reliable for achieving drainage of the distended afferent loop because the amount of drainage can be checked, which leads to the alleviation of obstructive jaundice and ascending cholangitis. Second, in the determination of the therapeutic strategy, it is important to evaluate whether the bilioenteric anastomosis is involved in the obstruction site. If the bilioenteric anastomosis is obstructed by tumor recurrence, double stenting is required to achieve decompression of both the afferent loop and the biliary duct. However, it is often difficult to diagnose the comorbid obstruction of a bilioenteric anastomosis by CT or ultrasonography. Contrast medium injection through the nasojejunal drain is helpful for assessing bilioenteric obstruction. Finally, evaluating the length and location of the obstruction site after decompressing the afferent loop enables a suitable SEMS placement. This approach may facilitate an accurate diagnosis as well as the selection of therapeutic strategies for ALO with ascending cholangitis.
All the patients in our series were treated using endoscopes of the conventional length. Recently, the use of balloon-assisted endoscopy during SEMS placement for malignant ALO has been reported [4,7,9,11-14,16]. Shimatani et al. reported a case in which SEMS placement was used to treat ALO caused by cancer recurrence after PD, in which a newly developed, shorttype, double-balloon endoscope (S-DBE) (EI-580BT; Fujifilm, Tokyo, Japan) was used [13]. They claimed that the combined use of the new S-DBE, which had a 3.2-mm working channel, and SEMS with a 9 Fr delivery system enabled through-the-scope SEMS placement, which had been previously challenging because the large diameter of the SEMS delivery system did not allow stent deployment through the 2.8-mm working channels of the conventional S-DBE.
If the stricture is long or an angulated loop, endoscopic SEMS placement across the stricture is challenging. Recently, endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ) using a lumen-apposing metal stent has been reported [17-19]. In addition, we developed a method involving a conventional SEMS with antimigration properties, comprising a largeloop double-pigtail plastic stent within a fully covered biliary SEMS [20]. While further studies are needed, EUS-GJ may be considered an alternative treatment for selected malignant ALO cases.
In conclusion, endoscopic SEMS placement may represent an effective and safe treatment for malignant ALO.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP19K07938.



