A New Active Locomotion Capsule Endoscopy under Magnetic Control and Automated Reading Program
Article information
Abstract
Capsule endoscopy (CE) is the first-line diagnostic modality for detecting small bowel lesions. CE is non-invasive and does not require sedation, but its movements cannot be controlled, it requires a long time for interpretation, and it has lower image quality compared to wired endoscopy. With the rapid advancement of technology, several methods to solve these problems have been developed. This article describes the ongoing developments regarding external CE locomotion using magnetic force, artificial intelligence-based interpretation, and image-enhancing technologies with the CE system.
INTRODUCTION
In 2001, the Food and Drug Administration (FDA) approved the application of small bowel (SB) capsule endoscopy (CE) [1], which was introduced to Korea the following year [2]. CE is a very useful diagnostic modality for detecting SB diseases including obscure gastrointestinal (GI) bleeding, Crohn’s disease, SB tumors, and polyposis syndrome [3,4]. An advantage of CE is that it is a non-invasive, non-sedative procedure and allows direct mucosal visualization [5]. However, gastric retention or delayed transition of CE may lead to incomplete SB examination and may not detect SB lesions [6]. Also, the interpretation of CE images usually takes more than an hour, which can be a tedious process for clinicians [7]. In addition, enhancement of the CE image quality is needed to improve the diagnostic accuracy. To resolve these problems, many studies have been conducted on active locomotion, artificial intelligence (AI)-based interpretation, and image enhancement of CE. This article reviews the newly developed diagnostic and interpretation technologies of CE.
ACTIVE LOCOMOTION UNDER MAGNETIC CONTROL
CE is moved by gut peristalsis and gravity [6,8]. So, there is a possibility of incomplete SB examination if the CE remains stagnant in a specific area of the stomach or SB [9]. Although a longer battery life improved the success rate of complete SB examination [10], incomplete SB examination has still not been solved. Moreover, there is a need for non-invasive gastric examinations with the advantages of CE, but gastric examination using CE is impossible due to the wide lumen of the stomach [11]. Therefore, many studies regarding active locomotion in CE have been conducted for complete gastric examinations and reductions of the pyloric transit time.
The active locomotion systems of CE are generally divided into internal and external locomotion [12]. Some studies have been conducted on the internal locomotion that controls CE movements by using paddling, legs, and propellers within the GI tract [12]. However, internal locomotion was problematic due to high power consumption and unstable movements, and it was impossible to integrate the advanced technology for internal locomotion in a small capsule [13]. Therefore, external locomotion using magnetic force has emerged as a feasible solution [14]. In 2010, human gastric examination with CE using magnetic manipulation was first published [15]. Since then, another study validated the use of an external magnetic controller for gastric examinations with CE [16]. An external magnetic controller manipulates CE movements through rotations, tilting, and jumping [17]. It significantly reduced the median pyloric transit time in the gastric examination (4.7 minutes vs. 58.4 minutes in study and control groups, respectively) [18]. Currently, magnetic capsule endoscopy (MCE) is the main trend of external locomotion, and complete stomach and SB examination is becoming possible. There are currently several leading companies that have developed MCE with active locomotion for gastric examinations [11].
Siemens Healthcare and Olympus Medical Systems Co.
Siemens Healthcare (Erlangen, Germany) and Olympus Medical Systems Co. (Center Valley, PA, USA and Tokyo, Japan) jointly developed an MCE system applying an electromagnetic coil that manipulates a joystick controller to navigate the stomach. In a study that utilized the MCE system for the gastric examinations of 53 participants, the feasibility of the MCE system was confirmed as the antrum, body, fundus, and cardia were fully visualized in 98%, 96%, 73%, and 75% of cases, respectively [19]. Moreover, another study was conducted to compare the effectiveness of this MCE system and esophagogastroduodenoscopy (EGD) in identifying gastric lesions, such as inflammation, polyps, and ulcers. Among the 61 patients, the MCE system missed 14 lesions, but EGD missed 31 lesions. The overall diagnostic accuracy was similar between the two methods, indicating the potential for MCE as a screening method in high incidences of gastric cancer [17]. However, it is not currently commercially available [20].
IntroMedic Co., Ltd.
Mirocam-Navi system, developed by IntroMedic Co., Ltd. (Seoul, Korea), applies novel technology that uses the human body as a conductive medium to transmit data from CE to the electrodes attached to the body [21]. IntroMedic Co., Ltd. developed a hand-held magnetic controller as a new navigation system. When a gastric examination was performed using the Mirocam with a hand-held magnetic controller (Mirocam-Navi system), the landmarks of the stomach were successfully visualized (88%–100%). Thus, the Mirocam-Navi system was verified to be feasible for gastric examinations [22]. In addition, a hand-held magnetic controller has fewer space restrictions compared to the large-sized computer navigation systems of other companies. Moreover, well-experienced endoscopists using the Mirocam-Navi system may be able to inspect the lesions more accurately and shorten the examination time [23]. The Mirocam-Navi system had satisfactory maneuverability, mucosal visualization, and patient tolerance in esophageal, duodenal, and stomach examinations [24]. When the Mirocam-Navi system and EGD were compared among 33 patients with suspected acute GI bleeding, the diagnosis accuracy of focal lesions was higher in the Mirocam-Navi system (40 vs. 25, p=0.02), and bleeding focus in the SB was more likely detected by the Mirocam-Navi system than by EGD (18%) [25]. In addition, when the efficacy of the Mirocam-Navi system and EGD alone were compared among 49 patients with recurrent or iron-refractory iron deficiency anemia, the Mirocam-Navi system, which can combine examinations of the upper GI and entire SB, was more likely to detect pathologic lesions than EGD alone (113 vs. 52, p<0.001) [26]. Overall, the Mirocam-Navi system has high diagnostic accuracy and patient-friendly comfort for upper GI examinations.
Ankon Technology Co., Ltd.
At first, manual magnetic controllers showed good performances, but in the initial stages, the magnetic force was insufficient for pyloric passage [16] and an animal study showed that robotic magnetic control was more accurate than manual control [27]. Therefore, Ankon Technology Co., Ltd. (Shanghai, China) developed a C-arm-shaped robotic magnet controller and examined the stomach of healthy volunteers. In this study, the good maneuverability, detectability, and safety of the robotic MCE system was confirmed [28]. In several studies conducted using the robotic MCE system, the robotic MCE system showed high diagnostic accuracy similar to EGD, but less discomfort than EGD [29,30]. Moreover, it demonstrated a high sensitivity in detecting superficial gastric neoplasias [31]. Based on the valuable outcomes of these studies, a large-scale gastric cancer screening study involving 3,182 asymptomatic patients was undertaken using the robotic MCE system. In this study, gastric cancer was diagnosed in 7 people (0.22%). Since this rate was similar in Korea and Japan (approximately 0.2%), the robotic MCE system can be used as a screening tool for gastric cancer [32]. Because the MCE system shows a good diagnostic detection rate for gastric lesions, it can be a good alternative for EGD in high-risk patients [33]. Furthermore, pediatric patients, who are unable to tolerate EGD, can safely undergo successful gastric examination with the robotic MCE system [34]. Owing to its high diagnostic performance and safety, the robotic MCE system for human gastric examinations is currently approved by the Chinese FDA [35]. Recently, a second-generation MCE has been developed, which has greater image resolution, shortened examination duration, and improved maneuverability compared to the first-generation MCE [36].
ARTIFICIAL INTELLIGENCE-BASED INTERPRETATION PROGRAM
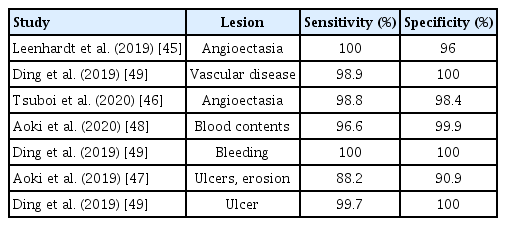
The interpretation of numerous SB images acquired by CE requires long processing times and high concentration from clinicians [37]. To solve this problem, a study was conducted to interpret CE images using a computer-aided diagnostic tool. This tool analyzed the images using characteristic colors of the lesions; however, it was difficult to use in real clinical practice because of its low sensitivity and specificity [38]. In a recent guideline, pre-interpretation by qualified nurses and trained technicians is recommended to reduce the burden and interpretation time on clinicians [39]. However, automated interpretation of CE has gained much attention with the development of AI technology, which had been firstly used in 1955 [40]. In the history of AI technology, the era of deep learning began in 2010 [41]. In 2012, the most well-known image recognition competition, ImageNet Classification, emerged. The first deep-learning model based on the convolutional neural network (CNN) model was showcased, and it significantly reduced the error rate compared to previous models [42]. The CNN model has presented outstanding outcomes in the field of medical image analysis and become the most preferred deep learning method in the field of medicine [43]. Several studies proved that the CNN model was superior to conventional machine learning models in terms of lesion analysis [44], so studies that utilized CNN-based interpretations for detecting SB lesions have been conducted since 2010. When CNN-based interpretations were used for the detection of angioectasia, the most common SB vascular lesion, it demonstrated excellent sensitivity and specificity close to 100% [45,46]. In addition, CNN-based interpretations showed an accuracy of 90.8% in the diagnosis of SB erosions and ulcers [47] and showed a higher diagnostic accuracy in blood contents (sensitivity, 96.63%; specificity, 99.96%) compared to conventional suspected blood indicators [48]. In a large-scale study in China, a new CNN interpretation model based on 113,426,569 images was developed, which showed a higher sensitivity (77.9%–99.9%) and lesion detection rate (54.6%–70.9%) compared to conventional CE interpretations for detecting various SB lesions, such as inflammation, ulcers, and polyps. The reading time was markedly shortened from 99.6 minutes to 5.9 minutes [49]. Important studies on CE interpretations using the CNN model are summarized in Table 1.
IMAGE-ENHANCING TECHNOLOGIES
It is important to improve the quality of images to increase the diagnostic accuracy of CE. Currently, the imaging enhancing methods of CE include 3D image reconstruction, capsule chromo-endomicroscopy, and improvements of the image resolution using de-noising and de-blurring processes.
A study showed that 3D reconstruction of the CE image may be helpful for inexperienced CE readers since elevated lesions observed in CE may be subepithelial lesions or normal variants [50]. IntroMedic Co., Ltd. recently commercialized a 3D CE equipped with a dual-stereo camera (MiroCam® MC 4000) which allows for 3D reconstruction (Supplementary video 1) and size measurements (Fig. 1). The entire SB examination was performed using this 3D CE, and its safety and feasibility were examined [51].

Image-enhancing technology for accurate identification of the lesions. The 3D reconstruction (A) and size measurements (B) by a Mirocam viewer (Miroview® MC 4000; IntroMedic Co., Ltd., Seoul, Korea).
Unlike wired endoscopy, CE cannot be used to perform biopsies for histopathologic diagnosis [6]. Thus, for optical biopsies, tethered capsule endomicroscopy (CE equipped with optics capable of obtaining cross-sectional images of the gut layer) was used [52]. In this study, 6 healthy patients and 7 patients with Barrett’s esophagus swallowed the tethered capsule, and the capsule was slowly pulled back from the stomach to the mouth for esophageal examination. The obtained 3D microstructural images accurately distinguished the normal mucosa and Barrett’s esophagus [52]. Capsule chromoendoscopy using flexible spectral imaging color enhancement improved the delineation of SB mucosal lesions compared with CE using conventional white light [53].
The most widely used CEs in the world, Pillcam SB3 (Medtronic Co., Ltd., Minneapolis, MN, USA) and Mirocam (IntroMedic), have a resolution of 320×320 pixels, while first-generation and second-generation MCE (Ankon Technology) have a resolution of 480×480 pixels and 720×720 pixels, respectively [31,35,54,55]. However, these CEs still have lower image resolutions than standard or high-definition wired endoscopies (400,000–1,000,000 pixels) [56]. CE cannot achieve a higher resolution due to the limitations of its wireless design and small size. Studies using de-noising and de-blurring algorithms are in development to obtain clearer images (Fig. 2).

Super-resolution by a Mirocam viewer (Miroview® MC 4000; IntroMedic Co., Ltd., Seoul, Korea). Erosion (A) and polyp (C) were noticed by a Miroview® MC 4000. A de-noising process, depth-guided deburring process, and deep-learning algorithm were used for super-resolution. Eventually, the clarity of the superresolution images (B and D) increased over the original images (A and C).
CONCLUSIONS
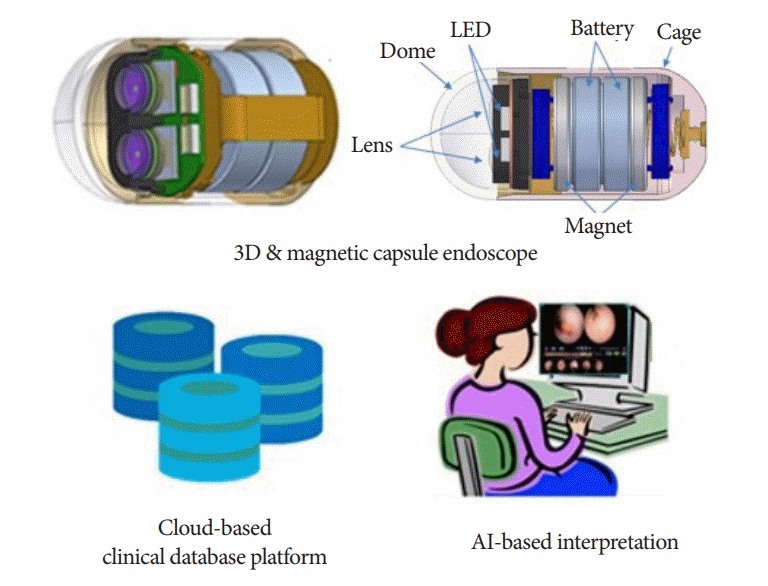
Magnetically controlled external locomotion and AI-based interpretation (especially CNN) allow for more efficient examinations of the GI tract. In addition, 3D reconstruction, high image resolution, and capsule chromo-endomicroscopy can elucidate the endoscopic and microscopic features of the lesions, thereby reducing unnecessary invasive procedures. If the MCE system with enhanced 3D imaging and AI-based interpretation are developed, whole gut (from the mouth to the anus) screening by a single capsule, pan-endoscopy will be possible in the near future (Fig. 3).
Acknowledgements
This study was supported by a grant (HI19C0665) from the Korean Health Technology R&D project through the Korean Health Industry Development Institute (KHIDI), Korea.
Notes
Conflicts of Interest: Kwang Seop Kim is working as a chief research engineer in the research and development team, IntroMedic Co., Ltd., Seoul, Korea. The other authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Yun Jeong Lim
Resources: Kwang Seop Kim
Writing-original draft: Dong Jun Oh
Writing-review&editing: YJL
Supplementary Material
Video 1.
The process of interpreting lesions by rotating the 3D reconstructed image (https://doi.org/10.5946/ce.2020.127.v001).