Clinical Practice Guideline for the Management of Antithrombotic Agents in Patients Undergoing Gastrointestinal Endoscopy
Article information
Abstract
Antithrombotic agents, including antiplatelet agents and anticoagulants, are increasingly used in South Korea. The management of patients using antithrombotic agents and requiring gastrointestinal endoscopy is an important clinical challenge. Although clinical practice guidelines (CPGs) for the management of patients receiving antithrombotic agents and undergoing gastrointestinal endoscopy have been developed in the Unites States, Europe, and Asia Pacific region, it is uncertain whether these guidelines can be adopted in South Korea. After reviewing current CPGs, we identified unmet needs and recognized significant discrepancies in the clinical practice among regions. This is the first CPG in Korea providing information that may assist endoscopists in the management of patients on antithrombotic agents who require diagnostic or elective therapeutic endoscopy. This guideline was developed through the adaptation process as an evidence-based method, with four guidelines retrieved by systematic review. Eligible guidelines were evaluated according to the Appraisal of Guidelines for Research and Evaluation II process, and 13 statements were established using a grading system. This guideline was reviewed by external experts before an official. It will be revised as necessary to cover changes in technology, evidence, or other aspects of clinical practice.
INTRODUCTION
Antithrombotic agents, including antiplatelet agents and anticoagulants, are actively used in clinical practice for primary and secondary prevention of cardio-cerebrovascular disease [1-4]. To date, various types of antiplatelet agents and anticoagulants have been developed, and new agents such as non-vitamin K antagonist oral anticoagulants (NOAC) are also being developed and released. With the aging of the population, the number of patients suffering from cardio-cerebrovascular disease and associated conditions is increasing in South Korea. Therefore, the number of patients receiving antithrombotic agents has increased in clinical practice.
Recent advances in equipment and techniques for endoscopy have enabled a wide range of endoscopic procedures for both diagnostic and therapeutic purposes. South Korea is a region with the highest incidence of gastric cancer in the world. Since the introduction of the National Cancer Screening Program, more than two-thirds of gastric cancers are diagnosed at an early stage, and nearly half of these patients are being treated with endoscopic resection [5-7]. Generally, the incidence of adverse events such as bleeding associated with diagnostic endoscopy is very low. In contrast, therapeutic endoscopic procedures have a higher risk of adverse events compared to diagnostic endoscopic procedures, and the use of antithrombotic agents can further increase the risk of adverse events. Decisions regarding the management of antithrombotic agents prior to endoscopic procedures should be made following a comprehensive assessment of the risk of thromboembolism due to the discontinuation of antithrombotic agents along with the risk of bleeding associated with endoscopic procedures [8,9].
Depending on the region and race, clinically available endoscopic instruments, endoscopic procedures, and risk of adverse events associated with endoscopic procedures may differ. The risk of developing thromboembolism caused by the discontinuation of antithrombotic agents also varies depending on the region and race. Therefore, clinical practice guidelines (CPGs) regarding the use of antithrombotic agents before and after endoscopic procedures should reflect local medical environments, and several guidelines have been developed till date [10-13]. Nevertheless, such guidelines have not yet been developed for the South Korean population. Given that it is uncertain whether the available CPGs will be suitable for patients in South Korea, we developed a guideline for the management of antithrombotic agents during the peri-endoscopic period. In addition to a comprehensive review of the literature, particular concerns were considered to develop a guideline that reflects the characteristics of epidemiology, clinical practice patterns, and medical resources in South Korea.
METHODS
Purpose and scope of clinical practice guideline
This CPG aimed to provide information on the management of antithrombotic agents during the peri-endoscopic period, based on a comprehensive review of current evidence and CPGs regarding bleeding and thromboembolic adverse events associated with endoscopic procedures in patients receiving antithrombotic agents. This CPG refers to adult patients who are taking antithrombotic agents for primary or secondary prevention of cardio-cerebrovascular disease and undergo diagnostic or elective therapeutic endoscopic procedures, excluding emergency endoscopic procedures such as endoscopic hemostasis. The target readers of this CPG are gastroenterologists who perform endoscopic procedures in primary, secondary, and tertiary care institutions. The CPG is intended to assist gastroenterologists in making decisions for appropriate treatments regarding the use of antithrombotic agents before and after endoscopic procedures. In addition, it aims to serve as a guide for resident physicians and healthcare workers, and to provide practical information for patients and the general public.
Organization of the clinical practice guideline committee and development process
The CPG committee including the former chairperson (Hoon Jai Chun), the former president (Soo Teik Lee and Ho Gak Kim), and executive committee members of the Korean Society of Gastrointestinal Endoscopy (KSGE), convened in November 2017. The members of the CPG committee established a strategy for the development of the CPG, appointed a director of the CPG project, and reviewed and approved the budget for this project. They reviewed suggested recommendations and ensured the editorial independence and participation of all parties involved in the development process. In March 2020, the CPG committee (the chairperson of the board of the KSGE, Joo Young Cho; the president of KSGE, Chan Guk Park; and the executive committee members of KSGE) reviewed the final version of the CPG and authorized its implementation.
The CPG committee organized the KSGE Task Force on CPG, which supervised the development of the CPG regarding the use of antithrombotic agents before and after endoscopic procedures. For the development of the CPG, Jeong-Sik Byeon, a board-certified gastroenterologist and the member of KSGE, was appointed as the director of the KSGE Task Force on CPG, and five other gastroenterologists (Seung Joo Kang, Eun Jeong Gong, Byung-Hoon Min, Cheol Min Shin, and Hyun Lim) participated as members of the KSGE Task Force on CPG. A CPG development methodology expert (Miyoung Choi) from the National Evidence-based Healthcare Collaborating Agency (NECA) collaborated on the development of the guideline. The KSGE Task Force on CPG selected key questions, conducted literature search, established recommendations, and drafted and revised the guideline.
The KSGE Task Force on CPG held a total of four meetings since December 22, 2017. The KSGE Task Force on CPG also held two workshops to set up a methodology for the development of the guideline and to review the development process (March 12, 2018 and November 10, 2018). These workshops involved training sessions on the methods of guideline development, grading of recommendation and level of evidence, and achievement of recommendation consensus. The KSGE Task Force on CPG chose the adaptation process and developed the CPG through online and face-to-face meetings.
Selection of the key questions
Selection criteria were made, and a questionnaire was developed through the population, intervention, comparison, outcome (PICO) process wherein key questions to be included in the CPG were derived. P (population) represents patients who have undergone diagnostic or elective therapeutic endoscopic procedures while taking antithrombotic agents, I(intervention) represents the interruption or replacement of antithrombotic agents during the peri-endoscopic period, C (comparison) includes the comparison group, which continues to use antithrombotic agents before and after endoscopic procedures, and O (outcome) represents the risk for the occurrence of adverse events associated with endoscopic procedures such as bleeding and thromboembolism. The members of the KSGE Task Force on CPG gathered key questions and specified the priority of each question to determine which questions were to be included in the CPG.
Literature search and selection of existing guidelines for adaptation
In May 2018, a literature search according to the key questions was performed using Ovid MEDLINE, KoreaMed, KoMGI, National Guideline Clearinghouse, and Guidelines International Network. The search index words included a combination of terms related to endoscopic procedures (“endoscopy” OR “esophagogastroduodenoscopy” OR “colonoscopy” OR “endosonography” OR “endoscopic retrograde cholangiopancreatography” OR “enteroscopy” OR “biopsy” OR “stent” OR “argon plasma coagulation” OR “papillary balloon dilation” OR “sphincterotomy” OR “fine needle aspiration” OR “percutaneous endoscopic gastrostomy” OR “ampullectomy” OR “cystgastrotomy” OR “pneumatic dilation” OR “polypectomy” OR “endoscopic mucosal resection” OR “endoscopic submucosal dissection”), terms related to antithrombotic agents (“antiplatelet” OR “platelet aggregation inhibitor” OR “aspirin” OR “acetylsalicylic acid” OR “thienopyridine” OR “clopidogrel” OR “prasugrel” OR “ticagrelor” OR “ticlopidine” OR “cilostazol” OR “triflusal” OR “anticoagulants” OR “warfarin” OR “coumadin” OR “heparin” OR “low molecular weight heparin” OR “enoxaparin” OR “dalteparin” OR “nadroparin” OR “non-vitamin K antagonist oral anticoagulant” OR “novel oral anticoagulant” OR “direct oral anticoagulant” OR “dabigatran” OR “apixaban” OR “rivaroxaban” OR “enoxaban” OR “bridge therapy” OR “antithrombin”), and terms related to CPG (“guideline” OR “recommendation” OR “practice guideline”).
The criteria for selecting existing CPGs for adaptation were as follows: (1) it should be evidence-based; (2) it should be published in Korean or English; (3) it should be presented between January 2000 and May 2018; (4) it should be a CPG for adults aged 19 years and above; (5) it should be the latest edition, if there are revised editions; and (6) it should be a CPG with external review and expert consensus. The exclusion criteria were as follows: (1) a CPG without a clear declaration of recommendations and evidence supporting recommendations; (2) an old edition of CPG that was a publication of a revised edition; and (3) a CPG developed by the adaptation process. Finally, four CPGs were selected for the evaluation and development of CPG (Fig. 1) [10-13].
For an update of the latest literature, studies published after 2016 were searched by combining and modifying search index words according to the key questions using MEDLINE and KoreaMed. The literature search was performed by a researcher working with the NECA, Miyoung Choi, and duplicate studies generated by cross searches among search engines were manually excluded. Two members were assigned to each key question, and they independently selected the literatures according to the established criteria. First, literature inappropriate for CPG development was eliminated by reviewing the titles and abstracts. In the case of studies not eliminated in this process, the decision to eliminate or select them was finalized after reviewing the entire study. In cases of disagreement between two members, study selection was determined by consensus. If consensus was not reached, the team leader made the final decision. The exclusion criteria for the latest literature were as follows: (1) studies not targeting humans; (2) studies not targeting patients relevant to the key questions; (3) studies not conducting interventions and comparative interventions related to the key questions; (4) studies presented only as abstracts, case reports, or reviews; (5) studies not published in Korean or English; and (6) studies that did not provide the original text. If there was an overlap of the study populations between studies, the one with the smaller size was excluded.
Risk of bias assessment, summary of evidence, and grade of recommendation
The quality of CPGs, which are subject to adaptation, was evaluated using the Appraisal of Guidelines for Research and Evaluation (AGREE) II. The AGREE II consists of 23 structured key items organized within six domains followed by two global assessment items [14]. Each of the AGREE II items are rated on a 7-point scale: 1, strongly disagree, to 7, strongly agree. Each of the selected CPGs was evaluated by three assessors, and a workshop for practicing and understanding AGREE II was held by a CPG development methodology expert, Miyoung Choi, to minimize inter-rater variability. Finally, four guidelines were selected based on the comparison of standardized scores of each category [10-13].
In addition to CPGs, validity of selected recent studies was assessed using consistent and systematic methods. The randomized comparative studies were evaluated using the Cochrane Risk of Bias [15], whereas the non-randomized studies were evaluated using Risk of Bias Assessment tool for Non-randomised Study (RoBANS) 2.0 [16]. Systematic reviews were evaluated using A MeaSurement Tool to Assess systematic Reviews (AMSTAR) [17]. The summary of evidence was determined using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method [18]. Randomized comparative studies were defined as having a high level of evidence, and observational studies were defined as having a low level of evidence. However, the quality levels of the studies were upgraded or downgraded in consideration of factors affecting the quality of the studies. The level of evidence was graded as follows: high, moderate, low, and very low.
The grade of recommendation was classified as strong or weak depending on the balance between benefit and harm of the recommendation, quality of evidence, values and preferences. A strong recommendation is directed to most patients because it has more positive than negative effects, is supported by high-quality evidence, and is highly valuable and more strongly preferred than other interventions [1]. A weak recommendation is also beneficial for a lot of patients although it has relatively small positive effects and/or weak-quality evidence. In the case of a weak recommendation, an alternative intervention method can be chosen depending on values and preferences of physicians. Recommendations, grades of recommendation, and levels of evidence are summarized in Table 1.
Review and approval of the guideline
The editorial committee consisted of 34 members of the KSGE Steering Committee and 14 members from the Insurance Committee. They conducted the review of the first draft with open-ended questions. The draft was revised by the KSGE Task Force on CPG and was reviewed again by the editorial committee to ensure the completeness of the guideline. As an external review of this guideline, a public meeting was held at “KSGE Days 2019”, in which endoscopists and nurses from across the country gathered on 16 November 2019. The final draft of guideline was revised and made based on the discussions during this meeting.
Provision of clinical practice guideline and plans for future updates
For wide provision and distribution of this CPG, the guideline will be co-published in Clinical Endoscopy (official journal of the KSGE) and the Korean Journal of Gastroenterology (official journal of the Korean Society of Gastroenterology). It will be posted on the website of the KSGE, and registered in the Korean Medical Guideline Information Center. Because the rapid distribution of the CPG to endoscopists through the databases is expected to be difficult, the KSGE will distribute the guideline for free through various channels including emails and will actively promote it at academic conferences, seminars, and workshops. This CPG will be revised as necessary to account for changes in technology, new data, or other aspects of clinical practice in the future.
Limitations
The most critical limitation of this CPG is the lack of local evidences in Korea. Evidences from foreign countries cannot be directly applied to the development of the guideline for the Korean population, because the risks of developing adverse events associated with endoscopic procedures and thromboembolism caused by withholding antithrombotic agents differ among countries. This CPG is not intended to provide absolute treatment standards in real clinical practice, but rather to help physicians make evidence-based clinical decisions with regard to the management of antithrombotic agents before and after endoscopic procedures. Therefore, the treatment for each patient should be finally determined by the physician considering various clinical factors of individual patients. This CPG cannot be used as a basis for health insurance to restrict physician’s practice or as a basis for a legal judgment on medical practice.
Editorial independence
This CPG was selected as a project of the KSGE and received financial support from the KSGE. However, the KSGE did not affect the process of the CPG development, and all members involved in the development of the CPG had no interest or potential conflicts of interest.
RISK STRATIFICATON OF PROCEDURES AND PATIENTS
Categorization of endoscopic procedures according to the risk of bleeding
This CPG categorized endoscopic procedures into low-risk, high-risk, and ultra-high-risk procedures, according to the risk of bleeding associated with endoscopic procedures reported in patients not taking antithrombotic agents (Table 2). Lowrisk endoscopic procedures were defined as procedures where the risk of bleeding was expected to be less than 1%. Those procedures included diagnostic endoscopic procedures with mucosal biopsy and therapeutic endoscopic procedures without mucosal incision. High-risk endoscopic procedures were defined as procedures where the risk of bleeding was expected to be more than 1%. Among high-risk endoscopic procedures, endoscopic mucosal resection (EMR) for large colon polyp (≥2 cm) and endoscopic submucosal dissection (ESD), which are frequently performed in Asian countries, including South Korea, and which have a higher risk of bleeding compared with other high-risk endoscopic procedures, were categorized as ultra-high-risk endoscopic procedures as per the Asian Pacific Association of Gastroenterology (APAGE)/Asian Pacific Society for Digestive Endoscopy (APSDE) guideline [13].
Categorization of patients according to the risk of thromboembolism
Antiplatelet agents
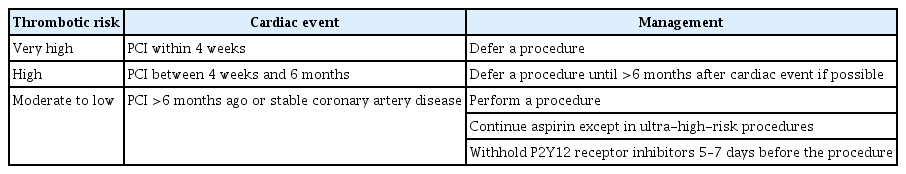
Patients who have undergone stent insertion due to coronary artery disease need to take antiplatelet agents such as aspirin or P2Y12 receptor inhibitors. Decision regarding the discontinuation of antiplatelet agents and the timing for executing high-risk endoscopic procedures should be made after comprehensive consideration of stent thrombosis, bleeding, and clinical problems that could occur secondary to delayed procedures. Nevertheless, there is limited direct evidence regarding the risk of major adverse cardiac events (MACE) such as stent thrombosis associated with endoscopic procedures in patients with coronary artery stents, and it is difficult to determine the optimal timing of procedures with a low risk of adverse events. The CPG for acute coronary syndrome proposed by the Korean Society of Interventional Cardiology in 2013 recommends consulting a cardiologist for the interruption of P2Y12 receptor inhibitors before elective non-cardiac surgery [19]. Patients who have undergone drug-eluting stent insertion for unstable angina or non-ST elevation myocardial infarction are recommended to postpone surgery if 12 months have not elapsed since stent insertion [19].
The CPG regarding dual antiplatelet therapy (DAPT) for coronary artery disease, which was developed by the American College of Cardiology/the American Heart Association in 2016, recommends delaying surgery for 6 months after inserting a drug-eluting stent and 30 days after inserting a bare metal stent [20]. In recent large-scale case-control studies, the prevalence of MACE was 7.2%–11.6% when surgery was performed within 4–6 weeks of coronary stent insertion [21-23]. Of note, a case–control study involving 9,391 patients showed that the type of stent was not associated with the risk of MACE [23]. Based on the results of these studies, the CPG for DAPT in coronary artery disease developed by the European Society of Cardiology (ESC) in 2017 recommends delaying surgery for 4 weeks after stent insertion, regardless of the type of coronary artery stents used [24]. In addition, when surgery is scheduled between 4 weeks and 6 months after stent insertion, surgery should be deferred if possible, and the decision to perform surgery should be made after considering the risks and benefits of the surgery specific to the patient [24]. Based on recent studies and previously developed CPGs for the use of antithrombotic agents before and after endoscopic procedures, we have summarized the recommendations regarding the timing of high-risk endoscopic procedures in patients who have undergone coronary stent insertion (Table 3).
Anticoagulants
Decisions to continue or discontinue anticoagulants in patients undergoing endoscopic procedures should consider both the risk of bleeding associated with endoscopic procedures and thromboembolism caused by withholding anticoagulants. The risk of thromboembolism, which may occur due to the discontinuation of anticoagulants, is closely related to the underlying disease requiring the use of anticoagulants, and the absolute risk of thromboembolism is known to increase by approximately 1% when anticoagulants are discontinued for 4–7 days [25,26]. However, most studies on the management of anticoagulants before and after endoscopic procedures are observational studies, and almost all CPGs and their recommendations are based on expert opinions. Moreover, recommendations are applied in various ways in real-world clinical practice, and there is no consensus even among experts. Regarding the risk of thromboembolism, the British Society of Gastroenterology (BSG)/European Society of Gastrointestinal Endoscopy (ESGE) guideline categorized patients into lowand high-risk groups according to the estimated annual risk of thromboembolism that may occur during anticoagulation discontinuation [11]. The American Society for Gastrointestinal Endoscopy (ASGE) guideline categorized patients into three groups according to the risk of thromboembolism: low-risk ( <5%), moderate-risk (5%–10%), and high-risk ( >10%) [12]. Based on recent studies and previously developed CPGs regarding the management of antithrombotic agents before and after endoscopic procedures, we summarized high-risk patients, for whom there is a high risk of thromboembolism when anticoagulants are withheld, requiring heparin bridging therapy (Table 4) [11-13].
RECOMMENDATIONS FOR THE MANAGEMENT OF ANTITHROMBOTIC AGENTS BEFORE AND AFTER ENDOSCOPIC PROCEDURES
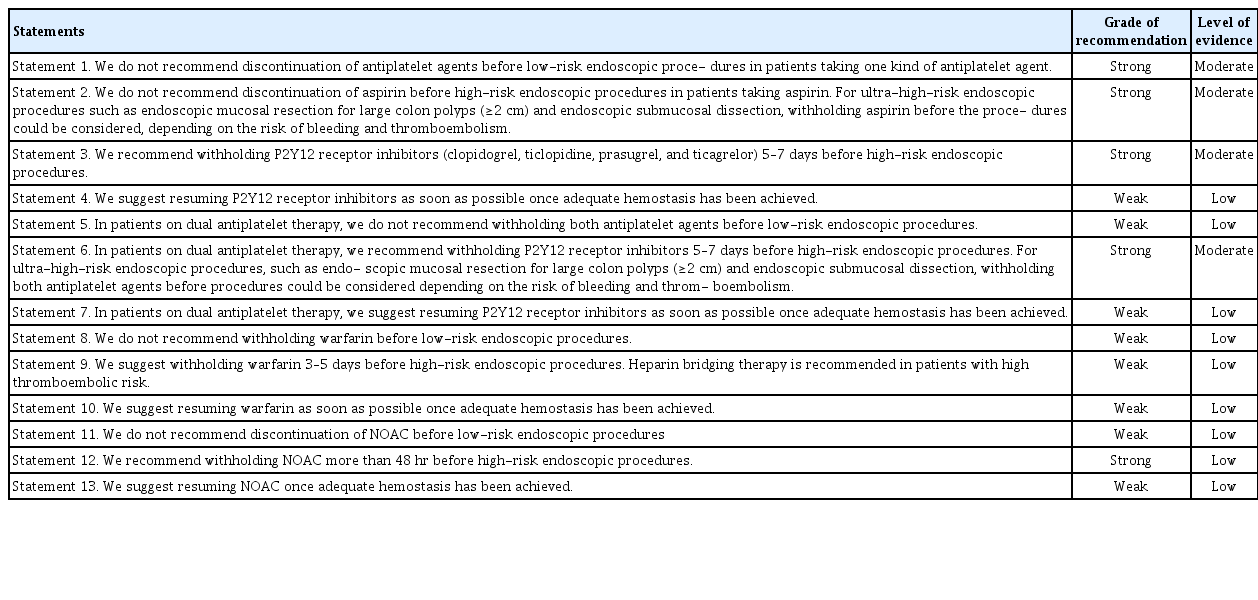
Statement 1: We do not recommend discontinuation of antiplatelet agents before low-risk endoscopic procedures in patients taking one kind of antiplatelet agent (Grade of recommendation, strong; Level of evidence, moderate).
The risk of bleeding associated with diagnostic endoscopy, including mucosal biopsy, is reported to be less than 0.1% even when an antiplatelet agent such as aspirin or clopidogrel is used [27-37]. A previous prospective study reported the bleeding rate after upper gastrointestinal endoscopy, including mucosal biopsy, without withholding antiplatelet agents before the procedure, and the bleeding rates in aspirin-only and clopidogrel-only groups were 0.4% and 0.0%, respectively [35]. In a Japanese prospective study involving patients taking antiplatelet agents, no bleeding occurred after upper gastrointestinal endoscopy or colonoscopy including mucosal biopsy [34]. Another retrospective study investigated the effect of antiplatelet agents on the bleeding rate after endoscopic papillary balloon dilatation in patients taking antiplatelet agents [38]. In that study, the bleeding rate was 0.8%, and no increase in bleeding rate was observed in the group that continued antiplatelet agents compared to that of the group that discontinued antiplatelet agents before the procedure.These results support the recommendation not to withhold antiplatelet agents before low-risk endoscopic procedures.
Statement 2: We do not recommend discontinuation of aspirin before high-risk endoscopic procedures in patients taking aspirin. For ultra-high-risk endoscopic procedures such as endoscopic mucosal resection for large colon polyps (≥2 cm) and endoscopic submucosal dissection, withholding aspirin before the procedures could be considered, depending on the risk of bleeding and thromboembolism (Grade of recommendation, strong; Level of evidence, moderate).
The prevalence of post-polypectomy bleeding (PPB) is reported to be 0.6%–2.2% [31,33,39-45]. Case–control studies of patients undergoing colonic polypectomy reported that aspirin use did not increase the risk of PPB [37,46-48]. In a Korean case–control study, the bleeding rate after colon polypectomy was 1.1%, and the frequency of aspirin use was not different between patients with PPB (n=92) and those without (n=276) [49]. A recent meta-analysis also showed that there was no increase in bleeding rate associated with colon polypectomy when aspirin was not withhold before the procedure (odds ratio [OR], 1.5; 95% confidence interval [CI], 0.9–2.2) [50]. Therefore, we recommend that patients who are taking aspirin alone do not need to withhold taking aspirin before colon polypectomy. However, if patients without underlying cardio-cerebrovascular disease are taking aspirin for primary prevention, withholding aspirin before the procedure may be considered.
The size of the colon polyp is a well-known risk factor for delayed bleeding after polypectomy. A previous study reported that the risk of delayed bleeding after colon polypectomy increased by 9% for every 1-mm increase in polyp size [51]. In particular, a higher rate of PPB has been reported after EMR for large colon polyps (≥2 cm) [52-54]. In an Australian study, the bleeding rate after EMR for large colon polyps (≥2 cm) was 7%, and the use of aspirin within 7 days before the procedure was associated with an increased risk of bleeding [54]. These findings suggest that discontinuation of aspirin could be considered before EMR of large colon polyps, depending on the risk of thromboembolism and procedure-related bleeding.
ESD carries a higher bleeding risk than EMR (OR, 2.20; 95% CI, 1.58–3.07) [55]. The bleeding rates after gastric and colon ESD were reported to be 3.6%–6.9% and 0.5%–9.5%, respectively [56-64]. While some studies reported that the risk of post-ESD bleeding was increased with persistent use of aspirin before ESD, others reported no increase in the bleeding rate regardless of aspirin discontinuation [56-64]. In a Korean study that investigated the bleeding rate after gastric ESD, the bleeding rates were 11.6% and 5.9% in the antiplatelet-continuation group and the withdrawal group, respectively; however, these rates were not statistically significant [61]. However, in another Korean study, the bleeding rate in the continuation group was significantly higher than that of the withdrawal group after gastric ESD (21.1% and 3.6%, respectively) [56]. Taken together, there remains controversy as to whether continuous administration of aspirin before ESD increases the risk of bleeding. Indeed, the BSG/ESGE and the APAGE/APSDE guidelines recommend withholding antithrombotic agents, including aspirin, before ESD because of the high risk of bleeding [12,13]. However, because the discontinuation of aspirin in patients taking aspirin for secondary prevention could increase the risk of thromboembolism, withholding aspirin before high-risk procedures should be determined based on the risk of thromboembolism and bleeding, ideally after consultation with a cardiologist or neurologist [65-67].
The bleeding rate associated with endoscopic papillary sphincterotomy (EST) has been reported to be 1%–5%, and an increase in bleeding rate was not observed even if antiplatelet agents were not discontinued before the procedure [38,68-74]. A Japanese retrospective study investigated the effect of antiplatelet agents on bleeding risk after EST in patients with choledocholithiasis [38]. In that study, the bleeding rate after EST was 0.8% in both the aspirin continuation and withdrawal groups. Another retrospective study reported that the use of aspirin within 7 days before EST did not increase the risk of bleeding associated with the procedure [73].
Statement 3. We recommend withholding P2Y12 receptor inhibitors (clopidogrel, ticlopidine, prasugrel, and ticagrelor) 5–7 days before high-risk endoscopic procedures (Grade of recommendation, strong; Level of evidence, moderate).
In patients taking P2Y12 receptor inhibitors, the risk of bleeding can be increased during high-risk endoscopic procedures such as colon polypectomy. In a prospective study that investigated patients undergoing colon polypectomy, the bleeding rate was 2.4% in patients who continued clopidogrel or prasugrel before the procedure, compared to 0.0% in patients who had never taken clopidogrel or prasugrel, showing a significant increase of the bleeding risk in the P2Y12 receptor inhibitor continuation group [75]. Recent studies including meta-analyses also reported that the continuation of clopidogrel increased the risk of bleeding after colon polypectomy (OR, 4.7; 95% CI, 2.4–9.2) [50,75-77]. Therefore, it is recommended to withdraw P2Y12 receptor inhibitors 5–7 days before colon polypectomy. The BSG/ESGE and APAGE/APSDE guidelines recommend temporary replacement with aspirin in patients who are expected to carry a higher risk of developing thromboembolism when discontinuing P2Y12 receptor inhibitors, after consultation with a neurologist or cardiologist [12,13].
A recent meta-analysis based on 74 studies showed that the use of an antiplatelet agent, including P2Y12 receptor inhibitors, was associated with a significant increase in the risk of bleeding after gastric ESD (OR, 1.63; 95% CI, 1.30–2.03) [60]. Otherwise, there are only a few studies regarding whether the continuation of P2Y12 receptor inhibitors is associated with the risk of bleeding after other high-risk endoscopic procedures [71,78,79].
Statement 4. We suggest resuming P2Y12 receptor inhibitors as soon as possible once adequate hemostasis has been achieved (Grade of recommendation, weak; Level of evidence, low).
Currently, there are no data supporting the ideal timing as to when to resume P2Y12 receptor inhibitors after high-risk endoscopic procedures. Therefore, it could be helpful to consult with a cardiologist or neurologist regarding the duration of discontinuation and the timing for resuming P2Y12 receptor inhibitors. In patients with a high risk of thromboembolism such as those undergoing coronary artery stent insertion, the risk of thromboembolism is increased if the duration of discontinuation of an antiplatelet agent is prolonged. Considering that it usually takes 3–5 days after resuming P2Y12 receptor inhibitors for the effect of the antiplatelet agent to appear, it is recommended to resume P2Y12 receptor inhibitors as soon as possible if adequate hemostasis is achieved during the procedure and there is no evidence of bleeding after the procedures [80]. Because resuming P2Y12 receptor inhibitors after high-risk endoscopic procedures may increase the risk of delayed bleeding, patient education and close monitoring are warranted.
Statement 5. In patients on dual antiplatelet therapy, we do not recommend withholding both antiplatelet agents before low-risk endoscopic procedures (Grade of recommendation, weak; Level of evidence, low).
There are few studies on the risk of bleeding associated with low-risk endoscopic procedures, including mucosal biopsy, in patients on DAPT. In a Japanese prospective study that analyzed 48 upper gastrointestinal endoscopies and 12 colonoscopies in 60 patients, including a total of 101 biopsies, there was no significant bleeding during 2 weeks after endoscopy (0/101; 95% CI, 0%–3.6%) [34]. In addition, the time until the bleeding stops on visual inspection after biopsy did not differ between patients taking a single antiplatelet agent and those on DAPT (2.4±1.4 and 2.1±2.1, respectively). However, the results of this study should be interpreted cautiously, because it was conducted in a small number of patients and lacked information on the use of histamine-2 receptor antagonists or proton pump inhibitors or the number of biopsies performed in each patient.
Statement 6. In patients on dual antiplatelet therapy, we recommend withholding P2Y12 receptor inhibitors 5–7 days before high-risk endoscopic procedures. For ultra-high-risk endoscopic procedures, such as endoscopic mucosal resection for large colon polyps (≥2 cm) and endoscopic submucosal dissection, withholding both antiplatelet agents before procedures could be considered depending on the risk of bleeding and thromboembolism (Grade of recommendation, strong; Level of evidence, moderate).
To determine the discontinuation of antiplatelet agents in patients on DAPT, the risk of bleeding associated with the procedure as well as the risk of thromboembolism should be considered simultaneously. In a meta-analysis of 161 patients with stent thrombosis after discontinuation of antiplatelet agents, the median time to stent thrombosis was 122 days when discontinuing P2Y12 receptor inhibitors alone and 7 days when discontinuing both aspirin and P2Y12 receptor inhibitors [80]. In a retrospective study of 1,385 patients undergoing colon polypectomy, the simultaneous use of aspirin and clopidogrel increased the risk of bleeding (OR, 3.7; 95% CI, 1.6–8.5) [77]. In that study, the delayed PPB rates of aspirin-only and DAPT groups were 1.0% and 3.5%, respectively (p<0.02). A recent meta-analysis also showed that the risk of bleeding after colon polypectomy was significantly increased when aspirin and clopidogrel were used simultaneously (OR, 3.4; 95% CI, 1.3–8.8) [50]. Therefore, we recommend withholding P2Y12 receptor inhibitors 5–7 days before the procedure in patients on DAPT, while continuing aspirin.
For ultra-high-risk endoscopic procedures, such as EMR for large colon polyps (≥2 cm) and ESD, the risk of bleeding associated with the procedures could be increased even when taking aspirin alone [52-54,56]. In a large-scale case–control study of 2,179 patients undergoing drug-eluting stent insertion in South Korea, the risk of MACE after discontinuation of both aspirin and P2Y12 receptor inhibitor was not significantly greater than that in patients who withheld only a P2Y12 receptor inhibitor, when the duration of discontinuation was less than 7 days [81]. These findings suggest that, for ultra-highrisk endoscopic procedures in patients taking both aspirin and P2Y12 receptor inhibitor, short-term discontinuation of the two antiplatelet agents could be considered depending on the risk of thromboembolism and bleeding after consultation with a cardiologist or neurologist.
Statement 7. In patients on dual antiplatelet therapy, we suggest resuming P2Y12 receptor inhibitors as soon as possible once adequate hemostasis has been achieved (Grade of recommendation, weak; Level of evidence, low).
There are no data on the optimal timing of resuming P2Y12 receptor inhibitors after high-risk endoscopic procedures in patients on DAPT. In patients with a high risk of thromboembolism or those with coronary artery stent, it is important to recognize that the risk of thromboembolism increases as the duration of P2Y12 receptor inhibitor discontinuation is prolonged. Therefore, it is recommended to restart P2Y12 receptor inhibitors as soon as possible once adequate hemostasis has been achieved and there is no evidence of further bleeding. Decisions on the duration of discontinuation and the timing for resuming P2Y12 receptor inhibitors should be individualized, and optimally after consulting a cardiologist or neurologist. In addition, since resuming of P2Y12 receptor inhibitors after the procedures could increase the risk of adverse events, including delayed bleeding, particular care should be taken when resuming P2Y12 receptor inhibitors.
Statement 8. We do not recommend withholding warfarin before low-risk endoscopic procedures (Grade of recommendation, weak; Level of evidence, low).
Few studies have investigated the risk of bleeding associated with low-risk endoscopic procedures, including mucosal biopsy, in patients on warfarin. The risk of bleeding associated with mucosal biopsy is considered very low, and it is considered safe to perform endoscopic biopsy while continuing antithrombotic agents [31]. A Japanese prospective study showed that there were no cases of delayed bleeding after biopsy in patients taking aspirin, clopidogrel, or warfarin, and that bleeding time after endoscopic biopsy did not differ between patients who took warfarin and those who did not [34]. Considering that the sequelae of thromboembolism are significant, it is recommended to continue warfarin where possible. However, because the risk of bleeding increases when the international normalized ratio (INR) exceeds the therapeutic range, it should be ensured that the INR is maintained within the therapeutic range during the peri-endoscopic period [82]. The APAGE/APSDE guideline recommends a delay of the endoscopic procedures if INR exceeds 3.5 prior to the procedures [13].
Statement 9. We suggest withholding warfarin 3–5 days before high-risk endoscopic procedures. Heparin bridging therapy is recommended in patients with high thromboembolic risk (Grade of recommendation, weak; Level of evidence, low).
There are limited data on the bleeding risk associated with high-risk endoscopic procedures in patients while continuing anticoagulants, and current guidelines on the periprocedural management of anticoagulants are largely based on expert opinions. Observational studies showed that the risk of bleeding associated with high-risk endoscopic procedures was significantly higher in patients taking anticoagulants than in those not taking them [46,83,84]. In a retrospective study investigating 1,657 colon polypectomies, warfarin therapy was an independent risk factor for PPB [46]. Previous studies have also assessed the methods to reduce the risk of bleeding associated with endoscopic resection when warfarin cannot be withheld because of a high risk of thromboembolism. When comparing cold snaring and conventional polypectomy for colon polyps less than 1 cm in patients without cessation of warfarin, delayed bleeding occurred in 0.0% (0/35) with cold snare polypectomy and in 14% (5/35) with conventional polypectomy (p=0.027), suggesting that cold snaring polypectomy can be employed in anticoagulated patients [85].
The INR decreased over 24–36 hours after discontinuation of warfarin, and the INR decreased to 1.5 in most patients after 3–5 days of warfarin discontinuation [86,87]. The risk of bleeding associated with endoscopic procedures does not increase when the level of INR is 1.5 or less. Therefore, it is recommended to withhold warfarin 3–5 days before high-risk procedures, and INR before and after the procedure should be checked. Recommendations for the required level of INR before high-risk endoscopic procedures differ according to the CPGs: the APAGE/APSDE guideline suggests that the procedures can be performed when the level of INR is 2.0 or less, whereas the BSG/ESGE guideline recommends endoscopic procedures to be undertaken if INR is below 1.5 [12,13].
During temporary interruption of warfarin therapy, heparin bridging therapy with unfractionated heparin or low-molecular-weight heparin is recommended in patients who are expected to have high risks of thromboembolism. The purpose of heparin bridging therapy is to reduce the risk of thromboembolism by minimizing the amount of time patients are not receiving therapeutic anticoagulation agents. The APAGE/APSDE, ASGE, and BSG/ESGE guidelines suggest indications for patients requiring heparin bridging therapy [11-13]. The guideline for stroke prevention in patients with nonvalvular atrial fibrillation proposed by the Korean Heart Rhythm Society recommends heparin bridging therapy only in patients with prosthetic valves [88]. It is based on a large-scale randomized controlled trial assessing the role of heparin bridging therapy in 1,884 patients with nonvalvular atrial fibrillation who needed warfarin interruption for invasive procedure or surgery, including 44.0% of gastrointestinal procedures [89]. In that study, the incidence of arterial thromboembolism did not differ between the bridging and no bridging groups (0.3% and 0.4%, respectively), while the risk of major bleeding was significantly higher in the bridging group than in the no-bridging group (3.2% and 1.3%, respectively). However, the proportion of patients with valvular heart disease ( ≤2%) or those with CHA2DS2 score of 5 or 6 ( ≤3.4%) was low, suggesting that these data cannot be applied to patients with a high risk of thromboembolism. Given the lack of definitive data on optimal treatment strategies for patients who require warfarin interruption, indications for heparin bridging therapy vary according to the CPGs. We suggest an indication of heparin bridging therapy by selecting a group of patients who are expected to obtain a greater therapeutic benefit of bridging while minimizing the risk of periprocedural bleeding (Table 4).
Statement 10. We suggest resuming warfarin as soon as possible once adequate hemostasis has been achieved (Grade of recommendation, weak; Level of evidence, low).
The timing for warfarin resumption should be determined based on both the risk of bleeding and thromboembolism. There are few studies supporting re-initiation of warfarin on the day of the procedure. In a study that analyzed 123 polypectomies for colon polyp less than 1 cm, bleeding that required transfusions occurred only in one case (0.8%) when resuming warfarin the day after the procedure [90]. In another study involving 109 colonoscopies, including hot biopsy and snare polypectomy, the bleeding rate associated with the procedure was 0.9% following resumption warfarin the day after the procedure [91]. Conversely, a study of 173 patients who underwent colon polypectomy showed that the risk of bleeding increased by about five times when resuming warfarin within a week following polypectomy [51].
Because it takes 5–7 days to reach the therapeutic range after re-administration of warfarin, we recommend early resumption of warfarin on the day of the endoscopic procedure in patients with high thromboembolic risk once adequate hemostasis has been achieved and there is no evidence of bleeding. The APAGE/APSDE guideline recommends resuming warfarin as soon as possible, and the ASGE and BSG/ESGE guidelines recommend resuming warfarin on the day of the procedure in patients with high risk for thromboembolism [11-13].
Statement 11. We do not recommend discontinuation of NOAC before low-risk endoscopic procedures (Grade of recommendation, weak; Level of evidence, low).
There is no evidence as to whether it is necessary to withhold NOAC before low-risk endoscopic procedures. The risk of bleeding associated with mucosal biopsy is considered very low, and endoscopic biopsy can be performed safely in patients taking anticoagulants [31,92]. The APAGE/APSDE and ASGE guidelines recommend against withholding NOAC before low-risk endoscopic procedures [11,13]. This is intended to minimize the risk of thromboembolism caused by discontinuation of anticoagulants. The Japan Gastroenterological Endoscopy Society guideline also recommends not to withhold NOAC before low-risk endoscopic procedures, and to perform the procedure at a time avoiding NOAC reaching its peak blood concentration [93]. In addition, they recommend minimizing the number of biopsies, confirming hemostasis before withdrawing the endoscope, and considering endoscopic hemostasis if bleeding does not stop spontaneously. By contrast, the BSG/ESGE guideline recommends withholding NOAC on the day of conducting low-risk endoscopic procedures [12]. Because the discontinuation of NOAC could increase the risk of thromboembolism, we recommend not to withhold NOAC before lowrisk endoscopic procedures.
Statement 12. We recommend withholding NOAC more than 48 hours before high-risk endoscopic procedures (Grade of recommendation, strong; Level of evidence, low).
Currently, four NOACs, including dabigatran (a thrombin inhibitor), apixaban, rivaroxaban, and edoxaban (a factor Xa inhibitor) are available in South Korea. There is no clear evidence as to whether it is necessary to withhold NOAC before high-risk endoscopic procedures. The Korean Heart Rhythm Society guideline recommends withholding NOAC at least 48 hr before invasive procedures, such as endoscopic procedures with a high risk of bleeding [94]. The APAGE/APSDE and BSG/ESGE guidelines also recommend withholding NOAC before high-risk procedures [12,13]. NOACs are characterized by rapid onset (1–4 hr) and offset (about 24 hr) of action. Because the half-life of NOAC is about 12 hr, it is predicted that NOAC levels will be almost undetectable after 48 hr. However, the metabolism of NOAC is affected by renal function. In particular, for dabigatran, about 80% of the drug is eliminated by the kidneys, and elimination is affected by renal function decline [95]. Therefore, special attention is needed for the management of NOAC in the context of impaired renal function [96]. In patients with normal creatinine clearance (CrCl), it is recommended to withhold NOAC in the 2 days preceding high-risk endoscopic procedures. For dabigatran, the duration of discontinuation before the procedure should be determined based on renal function: withhold for 3 days before the procedures if the CrCl is 50–80 mL/min and for 4 days before the procedures if the CrCl is 30–50 mL/min [97]. In brief, in patients using NOAC, renal function testing is required before high-risk procedures.
There is no evidence to support heparin bridging therapy in patients taking NOAC. The ASGE guideline recommends heparin bridging therapy only when NOAC cannot be resumed within 24 hr after endoscopic procedures [11]. Neither the APAGE/APSDE nor the BSG/ESGE recommends heparin bridging therapy during discontinuation of NOAC, based on its rapid onset of action [12,13]. The Korean Heart Rhythm Society also does not recommend heparin bridging therapy during temporary cessation of NOAC, because the anticoagulation effect of NOAC is predictable [94].
Statement 13. We suggest resuming NOAC once adequate hemostasis has been achieved (Grade of recommendation, weak; Level of evidence, low).
There are no data regarding the optimal timing of resuming NOAC after high-risk endoscopic procedures. Therefore, if necessary, it is best to consult with a cardiologist or neurologist regarding the duration of discontinuation and the timing for resumption of NOAC. The CPG of the APAGE/APSDE recommends early resumption of NOAC to minimize the risk of thromboembolism [13]. In contrast, the BSG/ESGE recommends resuming NOAC 24–48 hr after high-risk endoscopic procedures, considering bleeding risk and the rapid onset of action of NOAC [12]. Given that the longer duration of discontinuation of NOAC could increase the risk of thromboembolism, we recommend early resumption of NOAC once endoscopic hemostasis is achieved and there is no evidence of further bleeding.
CONCLUSIONS
The incidence of cardio-cerebrovascular disease is increasing with the aging of the population. This has led to an increase in the number of patients taking antithrombotic agents. The risk of bleeding varies according to the endoscopic procedures, and the use of antithrombotic agents could further increase the risk of such adverse events. To determine whether and when to withhold the use of antithrombotic agents before endoscopic procedures, the risk of thromboembolism caused by withholding of antithrombotic agents and the risk of bleeding associated with endoscopic procedures should be simultaneously considered. This CPG is intended to assist gastroenterologists who perform endoscopic procedures in decision-making and to provide a standard to guide clinical practice regarding the use of antithrombotic agents before and after endoscopic procedures. This guideline is expected to improve the safety and effectiveness of endoscopic procedures by minimizing adverse events such as bleeding and thromboembolism in patients using antithrombotic agents.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Jeong-Sik Byeon, Chan Guk Park, Joo Young Cho, Soo Teik Lee, Ho Gak Kim, Hoon Jai Chun
Data curation: Hyun Lim, Eun Jeong Gong, Byung-Hoon Min, Seung Joo Kang, Cheol Min Shin, Miyoung Choi
Formal analysis: HL, EJG, BHM, SJK, CMS
Methodology: MC
Supervision: BHM, JSB, CGP, JYC, STL, HGK, HJC
Validation: JSB
Writing-original draft: HL, EJG, SJK, CMS
Writing-review&editing: HL, EJG, BHM, SJK, CMS
Acknowledgements
We would like to express our gratitude to Dr. Miyoung Choi, a CPG development methodology expert from the Korean National Evidence-based Healthcare Collaborating Agency, who has provided invaluable advice for the development of the CPG.