Fatal Necrotizing Fasciitis Following Uncomplicated Colonoscopic Polypectomy: A Case Report
Article information
Abstract
Necrotizing fasciitis (NF) is a life-threatening infection that can be caused by various procedures or surgery and may develop in healthy elderly patients. Here, we report a case of a 66-year-old man with diabetes mellitus who underwent colonoscopic polypectomy, without complications. However, he visited the emergency department 24 hours after the procedure complaining of abdominal pain. Abdominopelvic computed tomography revealed multiple air bubbles in the right lateral abdominal muscles. After a diagnosis of NF was made, immediate surgical debridement was performed. However, despite three sessions of extensive surgical debridement and best supportive care at the intensive care unit, the patient died because of sepsis and NF-associated multiple-organ failure. In conclusion, physicians should pay special attention to the possibility of NF if a patient with risk factors for NF develops sepsis after colonoscopic polypectomy.
INTRODUCTION
Necrotizing fasciitis (NF) is a rapidly spreading, life-threatening infection that is characterized by extensive necrosis of the superficial muscle fascia and subcutaneous tissue, with mortality rates ranging from 29% to 100% [1,2]. The incidence of NF is relatively low at 0.4–2.8 cases per 100,000 individuals per year [3,4]. NF is notorious considering the high mortality rate, which increases considerably with the presence of predisposing factors such as diabetes mellitus (DM) [5-7]. Early diagnosis and surgical intervention are essential to reduce the mortality rate of this fatal complication. NF may be caused by various procedures such as intramuscular injection of nonsteroidal anti-inflammatory drugs [8,9], percutaneous endoscopic gastrostomy [10], percutaneous coronary revascularization [11], and transthoracic biopsy [12]. In addition, retroperitoneal NF has been reported after surgeries such as hemorrhoidectomy [13] and appendectomy [14]. However, currently, no case of colonoscopy-associated NF has been reported.
Here, we report a case of fatal NF that developed after an uncomplicated colonoscopic polypectomy.
CASE REPORT
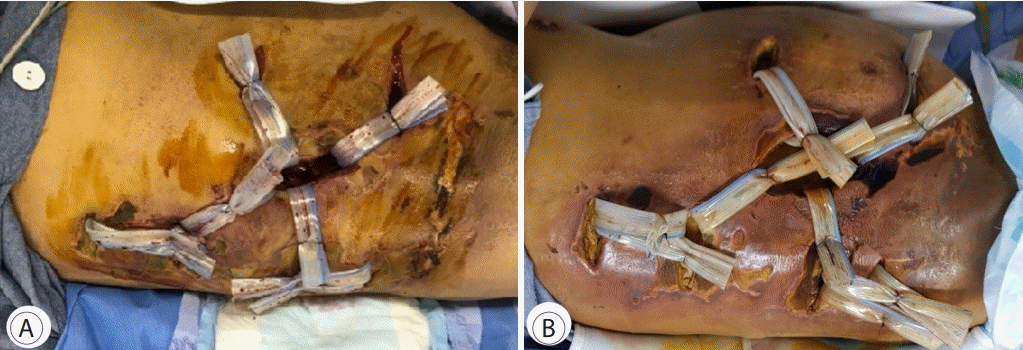
A 66-year-old man with DM and obesity (body mass index, 25.3 kg/m2) underwent colonoscopy after a positive fecal immunochemical test result. He had no history of trauma or surgery before colonoscopy. However, he did not know that he had DM, but his glycosylated hemoglobin level was 9.1%. He consumed a bottle of rice wine thrice a week. In the 58-minutes colonoscopic procedure, 15 polyps (size, 5–10 mm) were resected by endoscopic mucosal resection using a hot snare and preventive hemoclips were applied to the resection sites of large polyps to prevent delayed bleeding (Fig. 1). The distribution of polyps was as follows: nine polyps were located in the right colon (two in the cecum, four in the ascending colon, and three in the transverse colon) and six polyps were located in the left colon and rectum. The quality profile of colonoscopy was excellent, with a cecal intubation time of 3 minutes, as well as the quality of bowel preparation in all colon segments was excellent. He was discharged from the endoscopy room without complication or pain. However, he visited the emergency department 24 hours after the procedure complaining of right abdominal pain. Tenderness and rebound tenderness were noted upon physical examination of the right-side of the abdomen. The patient’s laboratory data obtained in the emergency department indicated severe infection, with the following findings: white blood cell count, 21,460/mm3 (reference range, 4,000–10,000/mm3); C-reactive protein level, 17.8 mg/dL (0–0.5 mg/dL); blood urea nitrogen level, 28 mg/dL (8–20 mg/dL); creatinine, 2.13 mg/dL (0.7–1.2 mg/dL); lactic acid, 2.4 mmol/L (0.5–2.0 mmol/L); and total bilirubin, 1.7 mg/dL (0.3–1.2 mg/dL). Abdominopelvic computed tomography (CT) did not show pneumoperitoneum or bowel perforation, but revealed multiple air bubbles in the right lateral abdominal muscles (Fig. 2). Peritonitis and air bubbles were not found in the peritoneal cavity but air bubbles were found in abdominal muscles; therefore, possibility of bowel perforation was excluded. Intensive medical treatments, including broad-spectrum antibiotic therapy (piperacillin/tazobactam, 4.5 g/day), were immediately started. After 20 hours of hospitalization, emergency exploratory laparotomy was performed at the surgeon’s discretion because the patient’s clinical condition, infection markers, renal insufficiency, and metabolic acidosis rapidly deteriorated. Laparoscopic right hemicolectomy was performed to determine the possible focus of infection in the abdomen based on CT findings, although no perforation was found in surgical and pathological findings. However, his condition aggravated rapidly with multiple-organ failure and metabolic acidosis. After consultation with the departments of infection, gastroenterology, and radiology, a diagnosis of NF of the abdominal wall muscle was made and urgent surgical debridement and drainage were performed 2 days after admission (Fig. 3). However, despite surgical debridement, extensive broad-spectrum antibiotic therapy, and best supportive care with renal replacement therapy in the intensive care unit, two more surgical debridements and drainages (Fig. 4) were performed because his condition did not improve and showed a wax-and-wane course. No bacterial growth was identified in multiple blood cultures and in the first surgical wound culture; however, imipenem-resistant Acinetobacter baumannii and extended spectrum beta-lactamase negative Escherichia coli were identified in the second surgical debridement. After 35 days of admission, he died because of septic shock and multiple-organ failure.

Abdominopelvic computed tomography scan showing swelling of the right lateral abdominal muscles with multiple air bubbles (white arrow and white triangle) in muscles. (A) Axial view, (B) coronal view.

(A) Gross findings of the abdomen, before the first surgical debridement, showing bullae formation and necrosis with dusky discoloration. (B) The first surgical debridement—performed with Penrose drainage and betadine-soaked gauze dressing.
DISCUSSION
This is the first case report of fatal NF that developed after colonoscopic polypectomy. Although the causal relationship between NF and colonoscopy polypectomy is unclear, particular attention should be paid to development of unexplained sepsis after colonoscopic polypectomy, especially in elderly patients with DM, obesity, and alcoholism. Successful management of NF depends on early diagnosis; however, the exact diagnosis may be delayed, like in this case, because NF is difficult to diagnose without direct visualization of the fascia, as the overlying tissue can initially appear unaffected. Optimal treatment should include an urgent surgical intervention because NF worsens despite administration of broad-spectrum antibiotics, like in our case. Microbiologically, NF is divided into the following four categories: polymicrobial infection (type I) [15]; monomicrobial infections such as those caused by Staphylococcus aureus (type II) [8]; monomicrobial infections such as those caused by clostridium species, gram-negative bacteria, or Vibrio species (type III) [3]; and fungal infection (type IV) [3]. Our case may be type I NF because type I NF occurs most frequently (55%–90%) in elderly patients with underlying DM [15]. The diagnostic clue may be the presence of gas in soft tissues on radiographic imaging, which is more likely to be type I or III NF [16].
In our case, the portal of entry was unclear because NF developed after uncomplicated colonoscopic polypectomy, as demonstrated by abdominopelvic CT and surgical findings and postoperative pathology. Furthermore, no form of trauma, including that caused by acupuncture and moxibustion, or operation history were noted. However, the possibility that the injection needle penetrated through the right colon during the endoscopic mucosal resection cannot be excluded. Major or minor trauma, skin breach, recent surgery, immunosuppression state, DM, malignancy, obesity, and alcoholism are known risk factors for NF [2,17]. However, NF can occur in healthy individuals of any age without a relevant medical history or clear portal of entry. Among the risk factors, DM is the most important comorbidity of NF, accounting for 52.1%–70.8% in the overall population of patients with NF [18-20]. Compared with NF patients without DM, those with DM were reported to have higher mortality and polymicrobial infection rates [20]. Our patient may have had a fatal clinical course because of uncontrolled DM.
In conclusion, NF may develop following colonoscopic polypectomy, especially in elderly patients with DM. Physicians should pay special attention to the possibility of NF if a patient with risk factors for NF develops unexplained sepsis after colonoscopic polypectomy.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding
None.
Authors’ contribution
Conceptualization: Jae Myung Cha
Investigation: Sang Youn Shin, Min Seob Kwak, Jin Young Yoon, Ha Il Kim
Writing-original draft: Min Kyu Chae
Writing-review&editing: MKC, JMC