Feasibility and safety of endoscopic submucosal dissection for lesions in proximity to a colonic diverticulum
Article information
Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) for diverticulum-associated colorectal lesions is generally contraindicated because of the high risk of perforation. Several studies on patients with such lesions treated with ESD have been reported recently. However, the feasibility and safety of ESD for lesions in proximity to a colonic diverticulum (D-ESD) have not been fully clarified. The aim of this study was to evaluate the feasibility and safety of D-ESD.
Methods
D-ESD was defined as ESD for lesions within approximately 3 mm of a diverticulum. Twenty-six consecutive patients who underwent D-ESD were included. Two strategic approaches were used depending on whether submucosal dissection of the diverticulum-related part was required (strategy B) or not (strategy A). Treatment outcomes and adverse events associated with each strategy were analyzed.
Results
The en bloc resection rate was 96.2%. The R0 and curative resection rates were 76.4% and 70.6% in strategy A and 88.9% and 77.8% in strategy B, respectively. Two cases of intraoperative perforation and one case of delayed perforation occurred. The delayed perforation case required emergency surgery, but the other cases were managed conservatively.
Conclusions
D-ESD may be a feasible treatment option. However, it should be performed in a high-volume center by expert hands because it requires highly skilled endoscopic techniques.
INTRODUCTION
In recent years, the prevalence of colonic diverticulosis has been increasing in both Western and Asian countries.1 Colorectal lesions are occasionally found in proximity to the diverticulum. These are generally considered pseudodiverticula and lack a muscular layer. Endoscopic resection (ER) is the first treatment of choice for colorectal lesions, with a negligible risk of lymph node metastasis. However, ER may be contraindicated for diverticulum-associated lesions due to the high risk of perforation.2 Therefore, surgical resection is often performed for such lesions, even if they can be managed via ER. Recently, endoscopic submucosal dissection (ESD) has become widely used as a promising en bloc resection technique for early stage colorectal lesions.3-5 ESD techniques have been improved and can be used in the management of lesions considered challenging for ER due to difficulties related to accessibility, such as those involving the appendiceal orifice and ileocecal valve.6,7 A previous study reported that treatment strategies based on lesion classification are useful for lesions near the appendiceal orifice (L-PAO).6 This is similar to cases of lesions associated with a colonic diverticulum. Therefore, this strategy for L-PAO might be applicable to ESD for lesions in proximity to a colonic diverticulum (D-ESD). Although some case reports and few studies on ESD for diverticulum-associated colorectal lesions have been reported, the feasibility and safety of ESD for lesions in proximity to a colonic diverticulum have not been fully clarified.8-15 Thus, the aim of the present study was to evaluate the feasibility and safety of D-ESD.
METHODS
Patients
Consecutive lesions in proximity to a colonic diverticulum that were treated with ESD were respectively reviewed at two tertiary referral centers in Kobe University Hospital and Kishiwada Tokushukai Hospital (Japan) between January 2010 and April 2020. Lesions located within approximately 3 mm of the diverticula were defined as proximity lesions in this study. All lesions were evaluated preoperatively for ESD indications using conventional endoscopy, followed by magnified chromoendoscopic examination. ESD indications were based on the Japan Gastroenterological Endoscopy Society (JGES) guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection.16 In the present study, the inclusion criteria were lesions which had no obvious deep submucosal invasion on preoperative endoscopic findings. Patients with other invasive cancers, inflammatory bowel disease, or familial adenomatous polyposis were excluded.
ESD procedure
All ESD procedures were performed under conscious sedation using flunitrazepam, dexmedetomidine, and pentazocine. A lower gastrointestinal endoscope PCF-260AI, PCF-H290T (Olympus Corp., Tokyo, Japan) or EC-L600ZP7 (Fujifilm Medical Corp., Tokyo, Japan) were used as the therapeutic scopes and a FlushKnife BT-S with a length of 1.5 mm or 2.0 mm (DK2620J-B15S or B20S; FTS, Tokyo, Japan) were used for surgical knife. A transparent hood (16675; TOP Corp., Tokyo, Japan) was affixed to the tip of the endoscope to achieve good visualization and allow stable submucosal dissection. For cases in which submucosal layer access was difficult, a short type ST hood (DH-28GR; Fujifilm Medical Corp.) and assisting tool, such as in the clip-flap method,17 were used to facilitate submucosal layer endoscopic entry. VIO 300D or VIO 3 (ERBE Elektromedizin, Tubingen, Germany) was used as an electrical power source. The electrical cautery settings were “endo-cut I mode” (effect: 2, duration: 3, interval: 3; and effect: 1, duration: 2 or 3, interval: 3) for mucosal incision and “forced coagulation mode” (effect: 2, 50 W and effect: 6.6) for submucosal dissection. Sodium hyaluronate (Muco Up 0.4%; Boston Scientific Japan Corp., Tokyo, Japan) was used as the injection solution to obtain adequate and sustained submucosal lifting. Carbon dioxide insufflation was used in all the procedures. In this study, all cases were performed by four expert endoscopists (experience with colorectal ESD >100 cases). In all cases where the diverticulum was dissected in D-ESD, the resulting muscle layer defect was sutured using endoclips after resection.
Classification according to the association between a lesion and a diverticulum
Lesions were classified into the following types (Fig. 1) based on the previous report on L-PAO.6
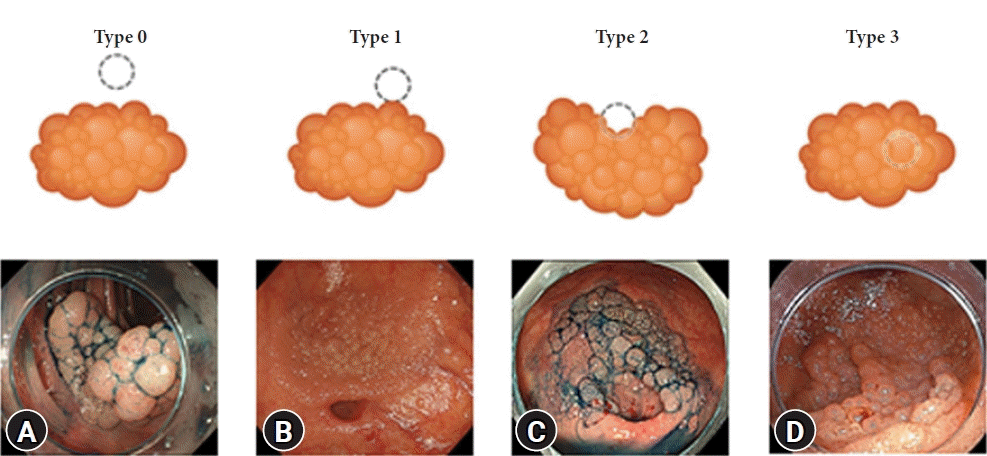
Classification according to the association between a lesion and a diverticulum. (A) Type 0: a lesion within 3 mm of the diverticulum border. However, a normal mucosa intervens between the diverticulum and the lesion. (B) Type 1: a lesion reaches the border of the diverticulum but does not involve the orifice of the diverticulum. (C) Type 2: a lesion reaches and partially involves the orifice of the diverticulum. (D) Type 3: a lesion completely covers the orifice of the diverticulum. In some cases, the presence of a diverticulum cannot be recognized preoperatively.
• Type 0: The lesion is within 3 mm of the border of the diverticulum, but a strip of normal mucosa intervenes between the diverticulum and the lesion.
• Type 1: The lesion reaches the border of the diverticulum but does not involve the orifice of the diverticulum.
• Type 2: The lesion reaches and partially involves the diverticulum orifice.
• Type 3: The lesion completely covers the orifice of the diverticulum. In some cases, the presence of a diverticula cannot be recognized preoperatively.
Basic ESD strategy according to lesion type
The following ESD strategies, namely, strategies A and B, were utilized according to lesion type (Figs. 2, 3). These strategies also conform to the ESD strategy for L-PAO. As needed, the clip-flap method17 was used to facilitate entry into the submucosal layer, and the traction method, which pulls the lesion upward, was used to facilitate submucosal dissection.

Strategic approach for endoscopic submucosal dissection of lesions in proximity to a colonic diverticulum. Strategy A for type 0 and some type 1 and type 3 lesions. (1) A semi-circumferential mucosal incision was made between the lesion and the diverticulum or from the anal side of the lesion. (2) Submucosal dissection was performed on the anal side towards the oral side. (3) A circumferential incision and submucosal dissection were performed in the remaining parts. Dot circle, diverticulum; red line, mucosal incision; blue arrow, submucosal dissection.
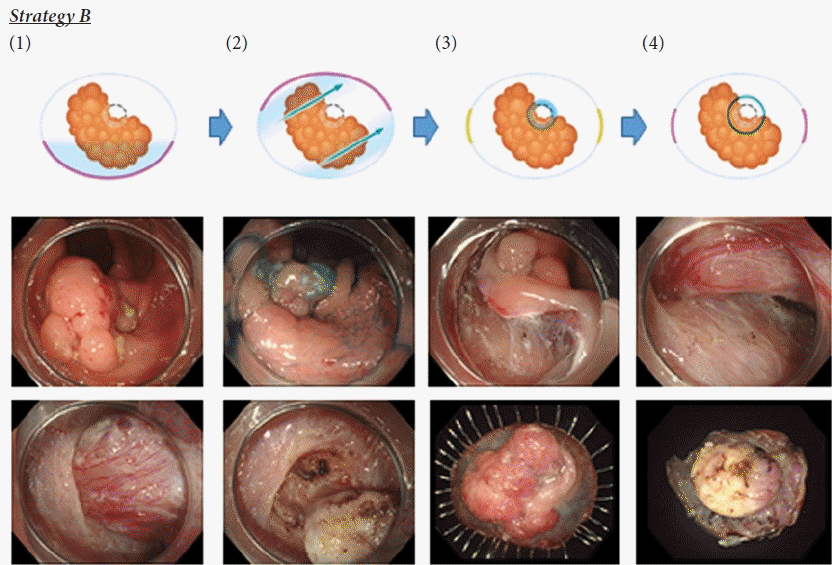
Strategic approach for endoscopic submucosal dissection of lesions in proximity to a colonic diverticulum. Strategy B for type 2 lesions and some type 1 and type 3 lesions. (1) A semi-circumferential mucosal incision was made from the anal side of the lesion. (2) Submucosal dissection was performed, and double pockets were made on both sides of the diverticulum towards the oral side. (3) Submucosal dissection around the diverticulum was performed maximally to expose the diverticulum under the lesion. Mucosae on both lateral sides were left at this time (yellow line). (4) Dissection of the diverticulum was performed carefully using the tapping technique, and the remaining mucosal incision was then completed. Dot circle, diverticulum; red line, mucosal incision; blue arrow, submucosal dissection.
• Strategy A: This method did not require submucosal dissection, including the diverticulum, and was indicated for the two types of lesions. One of them was when sufficient space to make an incision could be confirmed between the edge of the lesion and the orifice of the diverticulum (e.g., type 0, some type 1 lesions) (Fig. 2A). In this lesion type, a half circumferential mucosal incision was made between the lesion and the diverticulum. Submucosal dissection was then performed on the anal side toward the oral side. The remaining submucosal dissection was completed after making a circumferential mucosal incision. The other involved some type 3 lesions, wherein a sufficient submucosal layer was found between the lesion and the diverticulum (Fig. 2B). Strategy A involved a procedure similar to that of conventional ESD.
• Strategy B: This method required submucosal dissection, including the diverticulum (Supplementary Video 1). It was applied for lesions in which sufficient space to make an incision could not be confirmed between the edge of the lesion and the orifice of the diverticulum (e.g., type 2, some type 1, and type 3 lesions) (Fig. 3). The pocket creation method (PCM) was used for this strategy because it could provide stable endoscope maneuverability and good countertraction.18 First, a semi-circumferential mucosal incision was made from the anal side, and double pockets were made on both sides of the diverticulum. In addition, submucosal dissection around the diverticulum was performed to expose the diverticulum under the lesion. Notably, even after the double pockets were completed, the mucosa was left on both sides of the lesion. Thus, good countertraction could be obtained at the diverticulum. Then, submucosal dissection at the diverticulum was performed carefully using the tapping technique.19 Finally, the remaining mucosal incision was completed.
Definitions
En bloc resection was defined as treatment that was not discontinued during the procedure, and the lesion was resected in a single piece without fragmentation. R0 resection was defined as en bloc resection with histopathologically tumor-free lateral and vertical margins. A curative resection was indicated when R0 resection was achieved, and there was no submucosal invasion deeper than 1,000 µm from the muscularis mucosae, lymphovascular invasion, and poorly differentiated component. Among submucosal invasive carcinoma, T1a was defined as tumor invasion less than 1,000 µm from the muscularis mucosae and T1b as tumors that exhibited greater invasion, based on the Japanese classification criteria for cancer of the colon and rectum.16 Procedure time was started from the first submucosal injection until completion of the resection. The procedure speed was calculated by dividing the area of the resected specimen by the procedure time (mm2/min). The approximate area of the resected specimen (mm2) was calculated as follows: 3.14 × 0.25 × long axis diameter (mm) × short axis diameter (mm).20
Ethical statements
This study was approved by the Institutional Review Board (IRB No: B200312) and conformed to the provisions of the Declaration of Helsinki. Informed consent for study participation was obtained using an in-opt-out approach on the study website.
RESULTS
Characteristics of the patients and the lesions
Twenty-six patients with 26 lesions in proximity to a colonic diverticulum who underwent ESD were enrolled in the study. They consisted of 11 male patients and 15 female patients with an age range was 51–89 years old (median age, 70 years). Patient and lesion characteristics are shown in Table 1. Strategies A and B were used for the management of 17 lesions and 9 lesions, respectively. Most lesions (23/26; 88.4%) were located on the right side of the colon in relation to the distribution of diverticular development. The median tumor size was 33 mm (range, 15–115 mm). Three adenomas, two sessile serrated lesions, and 21 cancerous lesions were found. In cancerous lesions, 15 cases of intramucosal carcinoma, five cases of T1a, and one case of T1b were observed. The median diverticular size in all cases was 4 mm (range, 3–10 mm).
Treatment outcomes and adverse events
The treatment outcomes and adverse events of the treated lesions are summarized in Table 2. In one case in which strategy B was applied for type 3 lesions, ESD was discontinued because the operator judged that perforation risk was high due to deep tumor invasion to the diverticulum. All other patients achieved en bloc resection (96.2%). The R0 and curative resection rates were 76.4% and 70.6% in strategy A and 88.9% and 77.8% in strategy B, respectively. One case of lymphatic invasion was observed for each strategy. The clip-flap method was used in one case in strategy A and three cases in strategy B. The traction method was used in two cases in strategy A, but not in strategy B. The median procedure time of strategies A and B was 69 minutes (range, 24–140 minutes) and 100 minutes (range, 42–188 minutes), respectively. With regard to adverse events, two intraoperative perforations and one delayed perforation occurred in strategy A. In contrast, although no perforation was observed in strategy B, two post-ESD electrocoagulation syndrome (PECS) were noted.
The delayed perforation case required emergency surgery, but the other cases were managed conservatively. Two patients with deep submucosal invasion and lymphatic invasion and one patient who discontinued ESD underwent additional surgical resection. One patient underwent emergency surgery because of delayed perforation. Three patients had a postoperative observation period of less than one year and missing follow-up colonoscopy data. One patient died from another disease. The remaining 18 patients had a median follow-up of 24 months (range, 11–139 months), with no recurrence observed during follow-up.
DISCUSSION
The present study showed that ESD has favorable results when used in the management of lesions in proximity to a colonic diverticulum, which is often considered a contraindication to ER. The rates of en bloc and R0 resection were 96.2% and 80.8%, respectively. Lesions were classified into four types based on the positional relationship between the lesion and the diverticulum. In addition, two different strategies were applied based on the lesion type according to the previously reported classification for L-PAO.6 Type 3 lesions in which the diverticulum completely covers the lesion includes those that can be addressed with strategy A and those that require strategy B. In the present study, only those lesions that required submucosal dissection with excavation of the diverticulum were strictly distinguished as strategy B. Strategy B is expected to be more complicated and difficult than strategy A because it requires dissection of the diverticulum. Previous reports described ESD for lesions involving the diverticulum with a low R0 resection rate (33%).15 In contrast, all cases in which strategy B was applied for lesions involving the diverticulum achieved R0 resection, except for one case in which ESD was discontinued during the procedure. This difference in results may be due to the use of the PCM in strategy B. Yoshida et al.14 reported successful treatment of diverticulum-related lesions with ESD using PCM. It provides not only scope stability, but also good counter traction.18,21 In addition, strategy B provided better countertraction by creating double pockets on both sides of the diverticulum by leaving the mucosa on both sides. Recently, the traction method has been useful for ESD of colorectal lesions.22-24 One study and several case reports showed successful resection by ESD for lesions involving the diverticulum using the traction method.12,22,25 Traction toward the direction of pulling the lesion out of the diverticulum using traction methods is useful for safe dissection of the diverticulum. Muramoto et al.25 reported that traction is required in half of the lesions involving a diverticulum. In this study, the use of PCM in strategy B allowed for adequate exposure of the diverticulum and good countertraction of the diverticulum. As a result, in strategy B, R0 resection was achieved without using the traction method in all cases, including type 3 lesions, except for one case in which treatment was discontinued. The common point between the above report and this study is that application of countertraction to the diverticulum is necessary when the lesion involves the diverticulum. Selecting an appropriate strategy based on the lesion type and using the PCM for strategy B may improve the success rate of R0 resection in cases of such difficult lesions. As a matter of concern, type 3 lesions are often difficult to recognize preoperatively. These lesions are resectable with strategy A or require strategy B, depending on the extent of diverticulum involvement. If a diverticulum below the lesion was found during the procedure and was not identified preoperatively, it could be addressed by changing from strategy A to strategy B as needed. The diverticulum under the lesion was not recognized preoperatively in three cases; therefore, the strategy was changed from A to B during the procedure in this study. As a result, all cases achieved R0 resection. However, even with this method, complete removal of the lesion from the diverticulum is difficult in some cases because of the absence of any space for dissection between the lesion and the diverticulum (Supplementary Fig. 1). In such cases, if the diverticulum is outside the lesion, mucosal resection can be performed in the visible area. However, if the diverticulum is inside the lesion, treatment interruption and alternative treatment should be considered to avoid perforation. Jimenez-Garcia et al.15 reported that lesions involving the diverticulum that resulted in incomplete resection were those with a diverticulum larger than 6 mm in size. In contrast, strategy B in the present study included a case of diverticulum with a maximum diameter of 10 mm, which was also able to achieve R0 resection. Therefore, the morphology of the diverticulum may be more important than the size of the diverticulum in determining whether it is resectable. One of the major potential concerns in D-ESD is perforation. In particular, strategy B was expected to have a higher risk of perforation than strategy A because of a muscle layer defect in the diverticulum area after resection. In the present study, two cases of intraoperative perforation and one case of delayed perforation occurred in strategy A. In addition, the delayed perforation case was a recurrent lesion after ER and was accompanied by severe fibrosis. Pin-hole perforations occurred in both intraoperative perforation cases, which were immediately closed with endoclips. Postoperatively, the patient had mild fever and slight abdominal pain. However, both cases improved conservatively with fasting and antibiotic administration. In the case of delayed perforation, abdominal pain appeared on the day after ESD, and computed tomography examination showed free air; therefore, emergency surgery was performed on the same day. In contrast, no case of perforation was observed in strategy B. Several case reports have demonstrated that lesions involving a diverticulum can be treated without serious adverse events by closing the muscle layer defect after resection.9,12-14,25 In this study, the muscle layer defects were closed using endoclips for all cases in strategy B. No delayed perforation was observed in strategy B, but the incidence of PECS was high (2/9, 22.2%). When strategy B is performed, distinguishing PECS from delayed perforation is necessary; therefore, monitoring patients carefully for fever and abdominal symptoms postoperatively is important. Recently, full-thickness resection using an over-the-scope clip device (OTSC) has been reported for the treatment of lesions arising in a diverticulum.26,27 OTSC is a useful and less invasive treatment for lesions involving the diverticula but is limited to small tumors. Laparoscopic-assisted colorectal surgery is also a treatment option for lesions involving the diverticula. However, the results of this study, which involved mostly en bloc and R0 resection of D-ESD without severe adverse events, except for one case, suggest that ESD might be a less invasive treatment option for lesions that are close to a colonic diverticulum without evidence of deep invasion on preoperative examination.
This study has several limitations. First, it was a retrospective study with a small number of lesions. Second, all procedures were performed by skilled and experienced endoscopists. Colorectal ESD is technically more difficult than gastric or esophageal ESD because of poor endoscopic maneuverability and complications due to the anatomically thin wall. Furthermore, D-ESD, particularly strategy B, requires more precise and stable endoscopic manipulation. Therefore, the results of this study are not generalizable. Further investigation, including endoscopists with varying skill levels and a larger number of lesions, is warranted to elucidate the feasibility and safety of D-ESD. Third, most of the lesions in this study were located in the right-sided colon, and there were no sigmoid colon cases in strategy B. Asians, including Japanese, tended to have more colonic diverticula on the right side of the colon than Westerners who have more on the left side of the colon.28 This may be one of the reasons for the small number of lesions in the sigmoid colon in this study. The sigmoid colon is more tortuous and has a narrower lumen than the right-sided colon, which results in poor maneuverability of the endoscope. Therefore, ESD for lesions of the sigmoid colon may be difficult. Although curative resection was achieved in three lesions of the sigmoid colon resected with strategy A in this study, further investigation is needed to clarify whether strategy B is feasible for lesions of the sigmoid colon. Fourth, the follow-up period was short (24 months); therefore, a longer follow-up period is needed to elucidate the risk of recurrence. However, few comprehensive studies on ESD have been conducted for lesions associated with diverticula. Therefore, the results of this study support the benefits of D-ESD without increasing adverse events. Categorizing strategies according to lesion type may help endoscopists in selecting a more standardized strategic approach in strategy-planned D-ESD.
In conclusion, ESD for lesions in proximity to a colonic diverticulum by an expert endoscopist may be a feasible treatment option. However, it should be performed in a high-volume center because it is a highly technical procedure that requires extremely precise endoscopic manipulation.
Supplementary Material
Supplementary Fig. 1. A case in which the lesion could not be completely excavated from the diverticulum.
Supplementary Video 1. Endoscopic submucosal dissection for a lesion in proximity to a colonic diverticulum using strategy B (https://doi.org/10.5946/ce.2021.245.v001).
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2021.245.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: NI, TTo; Data curation: NI, TN, SU, TH, KM, TYa; Formal analysis: NI, KT, HS, HA; Supervision: ST, YK; Writing-original draft: NI, TYo; Writing-review & editing: TTo, SK, TTa, YM.


