Endoscopic diagnosis of gastric metastases from malignant melanoma: systematic review
Article information
Abstract
Background/Aims
Metastases of malignant melanoma (MM) are rare and associated with poor prognosis. The objective of this study was to analyze the clinical and endoscopic characteristics of gastric metastases of MM by systematically reviewing cases and case series involving patients diagnosed using upper gastrointestinal endoscopy.
Methods
The PubMed and LILACS databases were searched. Reports containing individual patient data were included. Outcomes such as clinical data, endoscopic findings, treatments, and survival were analyzed.
Results
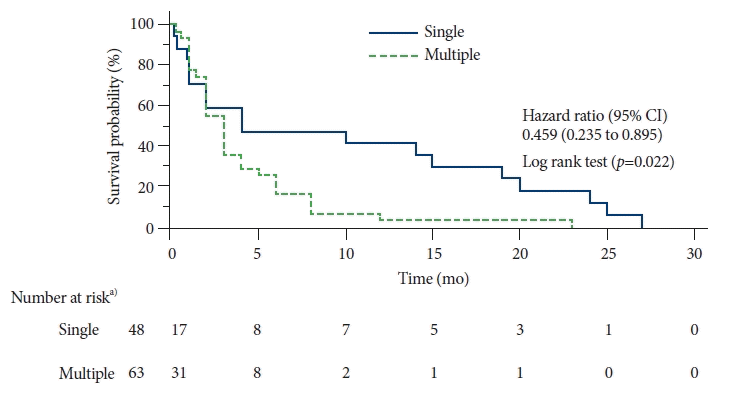
A total of 88 studies with individual data from 113 patients with gastric metastases of MM were included. The primary sites of MM were the skin (62%), eyes (10%), and mucous membranes (6%). Most patients (56%) had multiple metastases in the stomach, located predominantly in the gastric body (approximately 80%). The overall survival rate at 2 years was 4%. There was a significant reduction in the survival of patients with multiple gastric metastases compared to that of patients with single metastasis (hazard ratio, 0.459; 95% confidence interval, 0.235−0.895; p=0.022).
Conclusions
Gastric metastases of MM have a poor prognosis, especially in patients with multiple implants in the stomach. Additional studies are needed to verify whether ocular and mucosal melanomas are associated with a higher risk of gastric metastases than that of cutaneous melanomas.
INTRODUCTION
Malignant melanoma (MM) is an aggressive neoplasm that carries a significant risk of locoregional and distant metastases. The differential diagnosis between gastric metastasis of MM and primary melanoma of the stomach is performed based on the failure to identify the primary MM at another site. Primary melanoma of the stomach is rarer than gastric metastasis; a recent systematic review identified only 25 case reports describing primary melanoma of the stomach.1 Gastrointestinal involvement in cases of disseminated disease is common, especially in the small intestine. Secondary involvement of the stomach is rare and poorly described.2
The diagnosis of gastric metastasis of MM is often made through a postmortem.3 Few publications have described cases diagnosed using upper gastrointestinal endoscopy. Therefore, knowledge regarding the clinical characteristics, endoscopic data, treatment, and survival of this condition is limited.
The present study aimed to compile relevant data and analyze the clinical and endoscopic characteristics of MM gastric metastases by systematically reviewing cases and case series describing patients diagnosed using upper gastrointestinal endoscopy.
METHODS
Search strategy
We performed a systematic review in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).4 Case reports and case series of gastric metastases of MM diagnosed using upper gastrointestinal endoscopy were analyzed.
The searches were performed without restrictions on language or publication date. High-sensitivity searches were performed using the Medline/PubMed and Latin American and Caribbean Health Sciences Literature (LILACS) databases using the following MeSH terms: stomach neoplasms, melanoma, and gastrointestinal endoscopy. To identify additional eligible studies, the references of the included studies were manually searched.
After screening the titles and abstracts of the articles retrieved from the databases, the full texts of potentially eligible studies were examined by independent reviewers, and the inclusion criteria were applied. Disagreements between the reviewers were resolved by consensus.
Inclusion and exclusion criteria
Case reports and case series describing metastatic MM of the stomach, diagnosed using upper gastrointestinal endoscopy, were included. We excluded cases of primary stomach melanoma. Case series that did not present individual patient data were also excluded. If the same study was published more than once, the publications with more complete information were selected.
Data extraction
Individual patient data were extracted by independent reviewers using a predefined form. Clinical data such as those of age, sex, primary site of MM, metastases in other organs, and symptoms presented by the patients were collected. We also collected information on the number and location of lesions in the stomach. Finally, data on the treatments and their outcomes were obtained.
Data analysis
Medians or means and standard deviations were calculated for continuous variables, while frequencies and percentages were calculated for categorical variables. Student t-test was used to compare continuous variables, and chi-square test or Fisher exact test was used to compare categorical variables. For individual patient data that included survival time, survival curves were calculated by Kaplan-Meier analysis, and differences between the curves were analyzed using the log-rank test. Statistical analyses were performed using the MedCalc ver. 20.011 (MedCalc Software, Ostend, Belgium). Differences were considered statistically significant at p<0.05.
RESULTS
Study selection and population characteristics
The databases were searched from their inception to November 20, 2021, and 2,812 publications were identified. After screening the titles and abstracts, 2,652 articles were excluded. Twenty-six additional articles were identified by a manual search, resulting in 186 potentially eligible studies. After analyzing the full texts using the eligibility criteria, 88 articles remained for the analysis (Fig. 1).
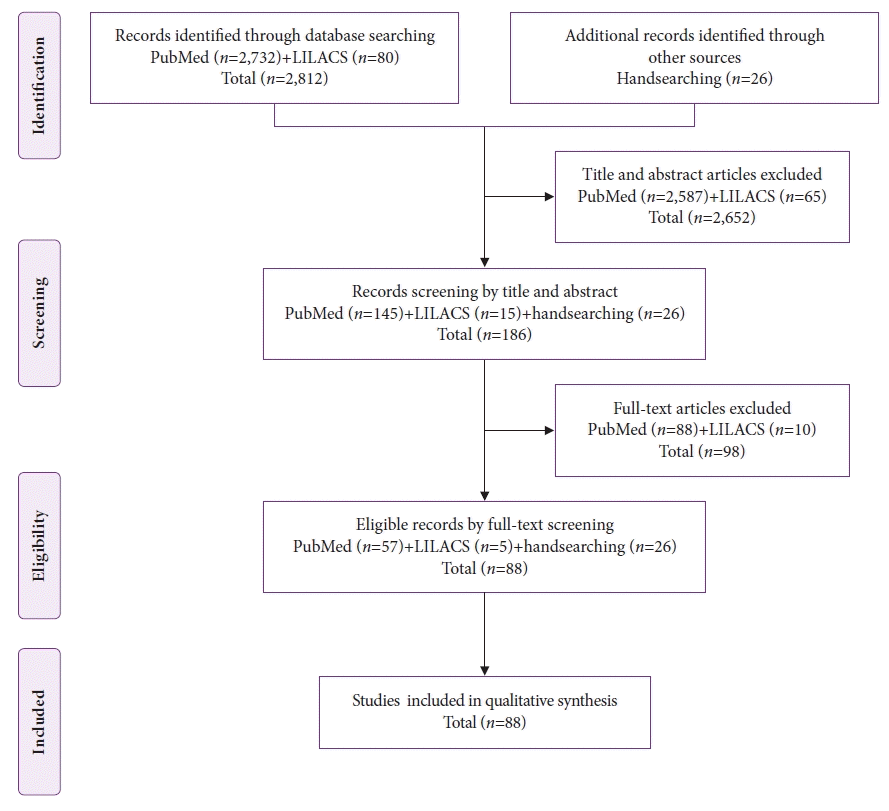
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection. LILACS, Latin American and Caribbean Health Science Literature.
Among the 88 included studies, eight were case series,2,3,5-10 and the other 8011-90 reported only one case each. Thus, the individual data of 113 patients with gastric metastases of MM were analyzed (Fig. 2).
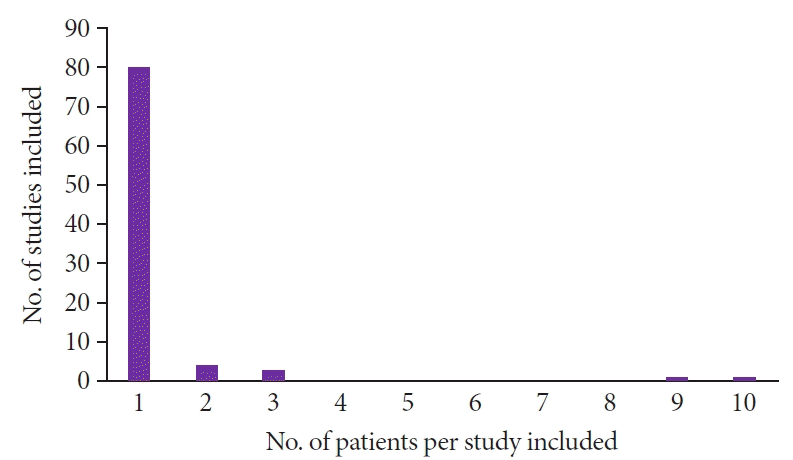
Among the 88 studies, 80 included one case. The other eight studies were case series. Four studies had two patients each, two studies had three patients, one study had nine patients, and one study had ten patients.
The patients’ ages ranged from 25 to 89 years (median, 63 years), and 64% of the patients were male. The most common symptoms were digestive bleeding (34.5%) and abdominal pain (34.5%).
The primary site of MM was reported in 78% of the patients and the skin was the most reported site (62%). Primary melanoma with an ocular location was described in 10% of the cases, and melanoma originated from the mucosal surfaces in 6% of the cases (Table 1).
Among the 113 patients with gastric metastases, 24 had metastases in the stomach only (21%) while 89 had metastases in other organs (79%) as well. The number of distant metastases in each of the 113 patients ranged from one to seven different sites (Table 2). In addition to the stomach, the other most affected organs were the lungs, liver, lymph nodes, duodenum, and central nervous system (Table 2).
A high percentage of the patients received chemotherapy (46%) or only palliative care (24%). Only 20 patients (18%) underwent surgery for gastric metastases.
Endoscopic findings
Single metastasis in the stomach was reported in 48 patients (42%) and multiple metastases were observed in 63 patients (56%). Data regarding the number of stomach metastases were not reported for two patients. Metastases was predominantly located in the gastric body (in approximately 80% of the cases). In only 5% of the patients, metastases were exclusively located in the gastric antrum (Table 3). The reports on the endoscopic characteristics of the lesions could not be validated as they varied widely, thus precluding the synthesis of these data.
Survival
In the present study, the overall 1-year survival rate was 16% and the 2-year survival rate was only 4%. The median survival time was 3 months. There was a significant reduction in the survival of patients with multiple gastric metastases compared to that of patients with single metastasis (hazard ratio for death, 0.459; 95% confidence interval, 0.235–0.895; p=0.022) (Fig. 3).
DISCUSSION
The present systematic review confirmed that patients with gastric metastases of MM have very low overall survival rate (2-year survival rate of 4%) and median survival of only three months. In addition, this study demonstrated that patients with single metastasis in the stomach had significantly higher overall survival than patients with multiple gastric metastases. A study by Kim et al.3 reported a similar result; the authors analyzed 37 patients with gastric metastases from several primary sites. Surgical treatment of MM metastases in the gastrointestinal tract is associated with increased survival.7 Thus, early detection followed by gastrectomy is key to obtaining better results.
According to a study involving more than 133,000 cases of MM, the prevalence of cutaneous melanoma, ocular melanoma, and mucosal melanoma was 94.9%, 3.7%, and 1.4%, respectively.91 However, in the present study, the prevalence of cutaneous melanoma, ocular melanoma, and mucosal melanoma was 61.9%, 9.7%, and 6.2%, respectively. Despite the small number of cases in the present review and the high percentage of unreported primary sites (22.1%), the hypothesis is that primary ocular and mucosal melanomas present a greater risk of dissemination to the stomach. Primary mucosal melanomas confer a high risk of distant metastases, ranging from 16.7% to 27.7%.92,93
Upper gastrointestinal endoscopy plays an important role in the diagnosis of gastric metastases. In 1962, Reed et al.90 published the first case of gastric metastasis of MM diagnosed using endoscopic biopsy. Before the emergence of modern upper gastrointestinal endoscopy, gastric metastases of MM were described as “bull’s-eye” or “target-sign” lesions on radiological examinations.94,95 However, this radiological imaging pattern may be found in several other diseases, such as lymphoma, carcinoma, Kaposi’s sarcoma, and other uncommon benign diseases.88
In 1978, Nelson and Lanza96 presented a proposal for an endoscopic classification system that consisted of three types of gastric lesions: (1) nodules varying in size, usually appearing to arise on the crest of the normal rugae, often ulcerated at the tip, and invariably melanotic; (2) raised, submucosal tumor-like masses with ulcerated centers which usually produced the so-called bull’s-eye configuration on radiologic examination, and did or did not exhibit melanin grossly; and (3) mass lesions with varying degrees of necrosis and melanosis. This classification system has been used by several authors.86,88 In 2001, Oda et al.97 presented a proposal for an endoscopic classification system of metastatic tumors of the stomach that included several primary sites. The lesions were classified into two main patterns: submucosal tumors (with or without central depression) or primary gastric cancer. Lesions similar to primary gastric cancer were subdivided into those similar to early gastric cancer and those similar to advanced gastric cancer. Finally, lesions such as advanced gastric cancer were classified as follows: type 1, polypoid; type 2, ulcerated with well-defined margins; type 3, ulcers with undefined margins; type 4, tumors with diffuse infiltration. Several authors have adopted this classification.3,6,30,98 In addition, elevated lesions without infiltration of the edges and with central ulceration are commonly referred to as volcano-like or donut-shaped. These various proposals for endoscopic characterization generated high variability among the included studies, which precluded the compilation of lesion characteristics in the present review.
An important endoscopic finding in this review was that of the high percentage of metastases located in the gastric body. This characteristic has been described earlier,99 and recently by other authors.3,97,100 The pathophysiological mechanism underlying the greater preference for involvement of the gastric body remains unknown.
A strength of the present study was that relevant data pertaining to an infrequent but highly severe condition were obtained. Using individual patient data, it was possible to construct and compare survival curves. The limitations of this review include the limited scope of the search, which was conducted in only two databases, and the retrospective nature of the cases reported in the included studies.
In conclusion, the spread of MM to the stomach is associated with poor prognosis, particularly in cases with multiple gastric metastases. Further studies are needed to assess whether ocular melanomas and mucosal surface melanomas are associated with a higher risk of gastric metastases than that of cutaneous melanomas. Finally, standardization of the macroscopic description of gastric metastases is necessary to facilitate better standardization of reports.
Notes
Ethical Statements
Not applicable.
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: HCR, ACAP, VFMMF, IPF, PMOC; Data curation: HCR, ACAP, VFMMF, IPF, PMOC; Formal analysis: HCR, ACAP, VFMMF, IPF, PMOC; Investigation: HCR, ACAP, VFMMF, IPF, PMOC; Methodology: PMOC; Project administration: HCR; Supervision: PMOC; Validation: PMOC; Visualization: HCR, ACAP, VFMMF, IPF, PMOC; Writing–original draft: HCR, ACAP, VFMMF, IPF, PMOC; Writing–review & editing: PMOC.