Endoscopic treatment for rectal neuroendocrine tumor: which method is better?
Article information
Abstract
Recently, research on rectal neuroendocrine tumors (NETs) has increased during the last few decades. Rectal NETs measuring <10 mm without atypical features and confined to the submucosal layer have only 1% risk of metastasis, and the long-term survival probability of patients without metastasis at the time of diagnosis is approximately 100%. Therefore, the current guidelines suggest endoscopic resection of rectal NETs of <10 mm is regarded as a safe therapeutic option. However, there are currently no clear recommendations for technique selection for endoscopic resection. The choice of treatment modality for rectal NETs should be based on the lesion size, endoscopic characteristics, grade of differentiation, depth of vertical involvement, lymphovascular invasion, and risk of metastasis. Moreover, the complete resection rate, complications, and experience at the center should be considered. Modified endoscopic mucosal resection is the most suitable resection method for rectal NETs of <10 mm, because it is an effective and safe technique that is relatively simple and less time-consuming compared with endoscopic submucosal dissection. Endoscopic submucosal dissection should be considered when the tumor size is >10 mm, suctioning is not possible due to fibrosis in the lesion, or when the snaring for modified endoscopic mucosal resection does not work well.
INTRODUCTION
Neuroendocrine tumors (NETs) originate from heterogeneous neuroendocrine cells and peptidergic neurons, and exhibit various biological behaviors according to anatomical sites and pathological features.1,2 The incidence of gastrointestinal NETs (GI-NETs) in Japan and the United States is reported to be similar (annual incidence rates of 2.10 per 100,000 and 2.53 per 100,000, respectively).3,4 However, marked differences in the distribution of GI-NETs have been observed between Asian and Western countries. In reports from the United States and Europe, midgut NETs occupy a large portion (38.7% in the United States, 30%–60% in Europe), and the small intestine is the most common site of GI-NETs. However, in Korea and Japan, the ratio of midgut NETs is low (the incidence of small intestinal NETs was only 7.7% in Korea, which is similar to 9.6% in a Japanese study), and the rectum was the most common site for GI-NETs in Korea.1,3 Recently, the number of rectal NETs and related clinical research has increased during the last decades due to advancements in endoscopic technology, such as endoscopy and imaging, the popularization of colonoscopy, and clinical endoscopists’ increased awareness of the disease.5-7 However, whether this increase is due to an increase in the detection rate of tumors or the incidence rate due to the widespread use of colonoscopy remains unclear. Most rectal NETs are more indolent than other epithelial malignancies although they can be aggressive and resistant to therapy. Rectal NETs are pathologically categorized according to the World Health Organization classification system, and their pathological grade is based on the mitotic and Ki-67 indices (Table 1).8
Although studies on rectal NETs are mostly retrospective with small sample sizes, and the efficacies of different treatment methods are still controversial with insufficient medical evidence, the treatment of this disease is gradually becoming standardized according to the proposal of corresponding guidelines.9,10 Rectal NETs are typically small (approximately 80% are <10 mm in diameter) single yellowish subepithelial lesions with intact overlying mucosa, not deeper than the submucosal layer, and are frequently located in the midrectum (within 10 cm from the anorectal junction).8 In a recent report of a series of 788 T1 rectal NETs, rectal NETs measuring <10 mm had 1% risk of metastasis, with the long-term survival probability of patients without metastasis at the time of diagnosis being 100%.11 Therefore, endoscopic resection is recommended for NETs <10 mm, because they have benign behavior and a low risk of metastasis in the absence of muscular and lymphovascular invasion.12,13 Meanwhile, for rectal NETs >20 mm, the metastatic risk is 60% to 80%; therefore, radical surgery and lymphadenectomy are recommended. There is an area of uncertainty regarding tumors between 10 and 20 mm, in which the metastatic risk is intermediate, and endoscopic treatment can be challenging. Since the risk of metastasis is approximately 10% to 15% for 10 to 20-mm NETs, the treatment method is determined according to endoscopic features, endoscopic ultrasound findings, grade, and muscularis propria invasion.14 Surgery, rather than endoscopic resection, should be considered when the NET size is ≥14 mm, an atypical endoscopic appearance indicates an ulcerofungating growth, central depression or ulcer, semipedunculated hyperemic color change, or muscular propria invasion is present on endoscopic ultrasound and magnetic resonance imaging of the pelvis.8,15
Therapeutic endoscopists select the endoscopic resection method according to tumor characteristics, such as size, morphology, and mucosal and submucosal appearance.16 The most important factor in predicting aggressive disease is the size of the primary tumor. Endoscopic resection of rectal NET can be divided into standard indications (tumor size <10 mm) and expanded indications (tumor size, 10–19 mm) according to tumor size. This review article describes which endoscopic resection method is the best standard modality according to each clinical situation.
ENDOSCOPIC RESECTION METHODS FOR RECTAL NEUROENDOCRINE TUMORS
Rectal NETs constitute approximately 1% of all rectal neoplastic lesions and are mostly asymptomatic.17 One key issue for endoscopic resection of rectal NETs is identifying them based on macroscopic features before improperly performing forceps biopsy, snare polypectomy, or conventional endoscopic mucosal resection (EMR). The complete resection rate was 68.2% when rectal NET was considered as a polyp before endoscopic resection, and 94.5% when diagnosed or suspected as NET before endoscopic resection.9,18 In addition, a preceding biopsy performed before endoscopic resection can interfere with complete resection by causing blurred tumor borders and fibrosis of the tissue.19 Therefore, the endoscopic findings of rectal NETs, such as smooth/round/sessile and yellow-discolored subepithelial nodules (reflecting the presence of chromogranin) <10 mm in diameter with intact overlying mucosa, typically observed within 5 to 10 cm from the anal verge, are strongly suggestive of rectal NETs and must be completely resected.
Endoscopic resection of rectal NETs should be aimed at en bloc and complete resection, as incomplete resection puts patients at risk for metastasis, resulting in repeated endoscopic and follow-up radiologic examinations and the need for salvage therapy.20 Various endoscopic resection methods, such as snare polypectomy, EMR, modified EMR (m-EMR), and endoscopic submucosal dissection (ESD), have been used to treat rectal NETs.
Conventional endoscopic mucosal resection
Complete resection rate of snare polypectomy is 20 to 30%.21,22 Conventional EMR proceeds snare cautery resection after lifting the lesion by submucosal injection to elevate the mucosal lesion away from the muscularis propria (Fig. 1A). Its advantages include being simple, less invasive, shorter procedure time (2–5 minutes), and a low complication rate (1.8%).23 However, similar to snare polypectomy, conventional EMR cannot adequately and completely resect lesions in the submucosal layer, and additional salvage interventions may be needed. Since 76% of rectal NETs extend into the submucosal layer,24,25 snare polypectomy and conventional EMR, which do not sufficiently capture the submucosal layer, are generally not selected because of the high risk of incomplete histologic resection. That's why it is not possible to sufficiently capture the submucosal part of the lesion by submucosal injection or snaring alone. Additionally, snare polypectomy and conventional EMR can cause crushing injury of the resection specimen, so it is disadvantageous for pathological evaluation, which is also why these methods are not commonly used.26 Several studies have reported that the complete resection rate of conventional EMR is 30% to 80%7,27-31 and an adverse event rate of 4.1%.28
Modified endoscopic mucosal resection
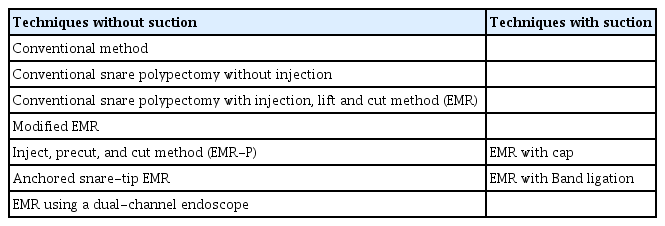
To overcome the limitations of standard polypectomy and conventional EMR, various m-EMR techniques have been utilized: EMR using a transparent cap (EMR-C); EMR with a ligation device (EMR-L), endoscopic submucosal resection with band ligation (ESMR-L); EMR using a dual-channel endoscope (EMR-D); EMR after circumferential incision/precutting (EMR-P), which is also called EMR with circumferential submucosal incision; and anchored snare-tip EMR (ASEMR). m-EMR has been classified in detail according to the specific procedure process. First, it can be divided into methods that use suction and those that do not. Methods using suction include EMR-C and EMR-L, whereas those that do not use suction include conventional EMR, EMR-P, ASEMR, and EMR-D (Table 2). The principle of m-EMR is to help the snare capture the deeper submucosal layer using suctioning and precutting. Thus, a higher complete resection rate is expected.
In EMR-C, the lesion is brought into the transparent cap by suction after submucosal injection. The lesion in the transparent cap is then captured with a snare to perform resection (Fig. 1B). EMR-C is technically easier and faster than ESD; therefore, it is an effective and safe technique for endoscopic resection of rectal NETs.32,33 The complete resection rate of EMR-C is reported to be 83.3% to 100%, the procedure time is 5 to 10 minutes, and the complication rate is 2.9% to 4.8%.7,30
First introduced in 1999, EMR-L uses suction with a transparent cap fitted to the scope, binding the bottom of the lesion with a ligation band, such as endoscopic variceal ligation, followed by closure of a snare beneath the ligation band so that it can be easily resected using a snare (Fig. 1C).34 Like EMR-C, EMR-L is technically easier and faster than ESD; therefore, it is an effective and safe technique for the endoscopic resection of rectal NETs. The complete resection rate of EMR-L is reported to be 95.5% to 100%, the procedure time is 5 to 10 minutes, and the complication rate is 0% to 4.8%.7,35 EMR-L with endoscopic ultrasonography is performed to confirm complete ligation of the lesion before snaring. Li et al.36 reported that ESMR with endoscopic ultrasonography demonstrated a slightly higher pathological complete resection rate than EMR-L (97.9% vs. 88.7%, p=0.152). They assessed this result as having practical clinical significance, although it was not statistically significant. Comparing EMR-C and EMR-L, EMR-L may be the preferable treatment method, considering both the endoscopic en bloc resection rate and histologic complete resection rate.7
EMR-P is performed by lifting the mucosa with a submucosal injection, making a circumferential incision/precutting along a margin that is 2 mm outside the tumor using the tip of the snare. Subsequently, the snare is securely positioned in the cut groove and tightened, and the tumor is resected using electrical current (Fig. 1D). The complete resection rate of EMR-P is reported to be 93.1% to 99.4%, the procedure time is 2.5 to 30 minutes, and the complication rate is 5.5%.37,38
In ASEMR, a small mucosal slit is made using the snare tip after submucosal injection, and then the snare tip is anchored into the mucosal slit, so it will not slip off the lesion (Fig. 1E). In comparison with EMR using suction, ASEMR achieves similar complete resection rates with minor complications. The complete resection rate of ASEMR is reported to be 94.1%, the procedure time is 2.8 minutes, and the complication rate is 6.7%.39 ASEMR has a shorter procedure time than EMR-P. In addition, compared to EMR using suction, there is the advantage that there is no need for a dedicated cap as in EMR-C or a band ligation device as in EMR-L.
EMR using a dual-channel endoscope is referred to as an EMR-D or strip biopsy. Recently, Lee et al.27 reported that the histological complete resection rate of EMR-D was 90.7%; this was significantly higher than that of conventional EMR (74.5%), and no significant differences were identified between EMR-L (93.1%) and EMR-P (90.9%). Sung et al.40 compared conventional EMR, EMR-D, and ESD in their prospective study. Although not significant (p=0.41), the histologic complete resection rate of EMR-D was 74.1%, which was lower than that of ESD (100%).
Endoscopic submucosal dissection
ESD is a minimally invasive advanced endoscopic technique used for en bloc and complete resection of GI tumors. ESD consists of the following three steps: submucosal injection to elevate the tumor, precutting the mucosa surrounding the tumor, and dissection of the connective tissue of the submucosa beneath the tumor (Fig. 1F). ESD has a high complete resection rate for rectal NETs. Several studies have reported the complete resection rate of ESD as 87.1% to 100%.29,31,38,40-43 Hybrid ESD is an effective and safe endoscopic resection technique for rectal NETs as an alternative to conventional ESD. Hybrid ESD involves a circumferential incision after submucosal injection similar to ESD. After circumferential incision, submucosal dissection proceeds to at least the bottom margin of the lesion, and then snaring is performed to excise the undissected lesion instead of using an endoknife. Recently, Wang et al.44 reported that hybrid ESD had a similar complete resection rate and safety profile as ESD, and the procedure time of hybrid ESD was shorter than that of ESD (complete resection rate 90% vs. 94.1%, mean procedure time 18.1 vs. 13.2 minutes, ESD vs. hybrid ESD, respectively). According to the endoscopist’s decision, changing the resection method from ESD to hybrid ESD can be considered if an appropriate vertical margin is secured during the procedure.
WHICH METHOD IS BEST?
Current guidelines recommend endoscopic resection for small rectal NETs that are <10 mm in size, with no atypical endoscopic appearance, and confined to the mucosa and submucosa.9,10 However, currently, there are no clear recommendations for the selection of any technique for endoscopic resection. As the options for endoscopic resection of rectal NETs have increased, a number of studies have been conducted to identify the methods with better outcomes. Data comparing different endoscopic methods used for the management of rectal NETs, including complete resection rates and complications, are presented in Table 3.7,27-31,35-42,44-48 Various factors should be considered to determine which method is better. Although a method with a high histologic complete resection rate is mandatory, the complication rate, status of the equipment in the hospital, proficiency of the therapeutic endoscopists and assistants, short procedure time, and length of hospital stay should be considered.
Snare polypectomy and conventional EMR are the easiest and simplest endoscopic resection methods, although they are not generally used as standard treatment because of the risk of incomplete resection. Zheng et al.45 reported that the odds ratio (OR) for histologic complete resection was 0.23 (95% confidence interval [CI], 0.10%–0.51%, p<0.01) when conventional EMR was compared to m-EMR. Kim et al.29 reported that the complete resection rate of conventional EMR was 77.4%, which was significantly lower than 97.7% of ESD. In a meta-analysis, Zhou et al.46 reported that the complete resection rate of conventional EMR was lower than that of m-EMR (relative risk, 0.72; 95% CI, 0.60%–0.86%) and ESD (relative risk, 0.89; 95% CI, 0.79%–0.99%). Therefore, snare polypectomy and conventional EMR are not considered standard options for endoscopic resection.
Both EMR-C and EMR-L are useful for removing rectal NETs <10 mm in diameter.42,43 In a recently published meta-analysis comparing the efficacy and safety of EMR with suction and ESD for small rectal NETs, EMR with suction was superior to ESD for small rectal NETs (≤10 mm) with a higher complete resection rate (OR, 4.08; 95% CI, 2.42–6.88, p<0.00001), shorter procedure time (standard mean difference, –1.59, 95% CI, –2.27% to –0.90%, p<0.00001), and similar overall complication rate (OR, 0.56; 95% CI, 0.28–1.14; p=0.11) and recurrence rate (OR, 0.76; 95% CI, 0.11–5.07; I2, 48%).47 In a study comparing ESD with EMR-L, EMR-L had a higher complete resection rate than ESD (95.5% vs. 75.0%, p=0.025).35 Additionally, one of the most important reasons for the superiority of EMR-L is its low vertical margin positivity rate. When the lateral and vertical margin distances from the tumor were measured, the lateral and vertical margins were more distant in the EMR-L group than in the ESD group (lateral margin distance, 1,661±849 vs. 1,514±948 μm; vertical margin distance, 277±308 vs. 202±171 μm, respectively).35 In another study measuring vertical margin distances from the tumor, EMR-L had a higher complete resection rate than ESD (100% vs. 85.7%) and a more distant vertical margin from the tumor in the EMR-L group than in the ESD group (vertical margin distance, 641.5±763.8 vs. 202.8±125.4 μm, EMR-L vs. ESD).48 In a study comparing the clinical outcomes of EMR-L and EMR-C, the endoscopic en bloc resection rate was higher in the EMR-L group (100% vs. 92.9%, p=0.003), although the complete resection rate was similar (92.5% vs. 83.3%, p=0.087, respectively).7 The reason EMR-L has a higher complete resection rate than EMR-C is that when using a band, lateral and deep margins are more easily secured in EMR, where EMR-L uses a technique that resects the tumor by snaring below the band ligation. Therefore, EMR-L may be the preferable treatment method relative EMR-C, because it is a simple and reliable procedure, regardless of operator skill.
EMR with suction cannot secure the resection margin when the lesion is larger than the cap or ligation band (for tumors ≥10 mm in size), which is limited because of the short diameter of the caps fitted to colonoscopies. In contrast, EMR-P has no size limitations for resection, because it captures the lesion by fitting the snare to the precut mucosa after circumferential incision/precutting.37 EMR-P has a complete resection rate that is comparable to that of EMR with suction. Several studies have reported that the complete resection rates of EMR-C, EMR-L, and EMR-P were 88.2% to 100%,30,31,39 88.7% to 100%,27-29,36 and 93.1% to 93.9%,37,38 respectively. ASEMR is a simple procedure that does not require a cap or ligation device. In a retrospective study, the histologic complete resection rate of ASEMR was 94.1%, which was not significantly different from that of EMR-C (88.2%).39 ASEMR had a shorter procedure time than EMR-C and a similar complication rate that was not significantly different from that of EMR-C.39 In this study, 11-mm and 12-mm rectal NETs were also completely resected using ASEMR with a 13-mm oval stiff snare, and their deep safety resection margins were 230 μm and 1,900 μm, respectively. Unfortunately, studies on ASEMR for rectal NETs are limited, although there are studies showing that ASEMR increases the complete resection rate and specimen size49-51; thus the potential se of ASEMR in the future may be promising. Therefore, EMR without suctioning (EMR-P and ASEMR methods) using only a simple injector and snare is also useful for the resection of small rectal NETs <10 mm.
ESD results in high en bloc and complete resection rates for rectal NETs. However, ESD has the disadvantage that the technique is difficult to learn, the procedure time is long, and the risks of bleeding and perforation are higher than those of m-EMR.27,29,40,43,45,46 Yong et al.41 reported a perforation rate of 2% and a bleeding rate of 7%. Incomplete resection and complications may occur if ESD is performed by an inexperienced operator. In addition, it has the disadvantage of requiring an expensive endoknife. In terms of complete resection, the effects of m-EMR and ESD are equivalent. Kim et al.29 compared the complete resection rate of EMR-L with that of ESD, and no significant difference was observed (EMR-L 100%, ESD 97.7%, p=1.000). Zhao et al.31 compared the outcomes of EMR-C with ESD in a retrospective study and reported that the complete resection rates of both EMR-C and ESD were 100%. In a meta-analysis, Pan et al.47 reported that EMR with suction for treating small rectal NETs (≤10 mm) had a higher complete resection rate than ESD (OR, 4.08; 95% CI, 2.42–6.88; p<0.00001). Chen et al.38 compared EMR-P with ESD, and the histologic complete resection rates were 93.9% and 96.4% (p=1.000), respectively, indicating no significant difference. Nevertheless, ESD is required if the lesion is too large, suctioning is not possible due to fibrosis in the lesion, or if the snaring for EMR does not work well. In a meta-analysis including 1,360 lesions, Yong et al.41 reported that ESD for rectal NETs >10 mm demonstrated a higher complete resection rate and lower vertical margin involvement than EMR.
CONCLUSIONS
The appropriate treatment for rectal NETs should be selected in consideration of the lesion size, endoscopic characteristics, proliferative index, grade, depth of vertical involvement, lymphovascular invasion, and risk of metastasis. Moreover, the complete resection rate, complications, and experience of the center should be considered when selecting an endoscopic method. m-EMR is the most suitable resection method for rectal NETs <10 mm in size, because it is an effective and safe technique that is relatively simple and less time-consuming than ESD. ESD is required when the tumor size is larger than 10 mm, suctioning is not possible due to fibrosis in the lesion, or when the snaring for m-EMR does not work well.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: DHB; Data curation: SMH, DHB; Formal analysis: SMH, DHB; Supervision: DHB; Writing–original draft: SMH, DHB; Writing–review & editing: SMH, DHB.