Optimal endoscopic drainage strategy for unresectable malignant hilar biliary obstruction
Article information
Abstract
Endoscopic biliary drainage strategies for managing unresectable malignant hilar biliary obstruction differ in terms of stent type, drainage area, and deployment method. However, the optimal endoscopic drainage strategy remains unclear. Uncovered self-expandable metal stents (SEMS) are the preferred type because of their higher functional success rate, longer time to recurrent biliary obstruction (RBO), and fewer cases of reintervention than plastic stents (PS). Other PS subtypes and covered SEMS, which feature a longer time to RBO than PS, can be removed during reintervention for RBO. Bilateral SEMS placement is associated with a longer time to RBO and a longer survival time than unilateral SEMS placement. Unilateral drainage is acceptable if a drainage volume of greater than 50% of the total liver volume can be achieved. In terms of deployment method, no differences were observed in clinical outcomes between side-by-side (SBS) and stent-in-stent deployment. Simultaneous SBS boasts a shorter procedure time and higher technical success rate than sequential SBS. This review of previous studies aimed to clarify the optimal endoscopic biliary drainage strategy for unresectable malignant hilar biliary obstruction.
INTRODUCTION
Malignant hilar biliary obstruction (MHBO) is caused by various types of malignant tumors. Most patients with MHBO are unsuitable candidates for curative surgery and have a poor prognosis because of their advanced disease stage at diagnosis.1 Therefore, palliative endoscopic or percutaneous biliary drainage is necessary to improve the obstructive jaundice caused by MHBO. A meta-analysis and systematic review reported that, compared to endoscopic biliary drainage (EBD), percutaneous transhepatic biliary drainage (PTBD) for MHBO was associated with a higher technical success rate and similar adverse effect and 30-day mortality rates.2 However, PTBD is an invasive procedure associated with several complications, patient discomfort, and decreased quality of life, particularly in patients with unresectable tumors who require prolonged percutaneous tube placement. A systematic review reported a significantly lower incidence of seeding metastasis in EBD than that in PTBD.3 Therefore, EBD is commonly performed as a first-line procedure because it is less invasive than PTBD. Endoscopic ultrasonography (EUS)–guided biliary drainage is an alternative drainage method that has recently been used to treat MHBO. In addition, EUS-guided hepaticogastrostomy, EUS-guided hepaticoduodenostomy, and bridging through the EUS-guided hepaticogastrostomy route can be used to facilitate MHBO drainage. However, these techniques are difficult to perform and evidence supporting their use are lacking.4,5
Endoscopic techniques for the management of unresectable MHBO vary in terms of stent type, drainage area, and deployment method. Consensus is lacking on the optimal drainage method because of limited data from randomized controlled trials (RCTs) for endoscopic drainage of MHBO versus malignant distal biliary obstruction. This is because patients with MHBO have more variable hilar stricture etiologies and severities than patients with malignant distal biliary obstruction. Here we evaluated the optimal biliary drainage strategy for unresectable MHBO.
STENT TYPE
Plastic stents
Plastic stents (PS) are commonly placed across the papilla for EBD of benign and malignant biliary strictures. PS are easy to place in MHBO because they do not obstruct the side branches of the intrahepatic bile duct near the hilar lesion. The advantages of PS include low cost and easy removability. The disadvantage of PS includes short stent patency (1–2 months) because of its small diameter.6 PS are commonly placed in patients who are expected to survive for at least 3 months or undergo surgery.7,8
PS placed above the papilla (inside the stent) have longer stent patency than PS across the papilla. Four studies evaluated the outcomes of internal stents. Inatomi et al.9 and Ishiwatari et al.10 reported median stent patency durations of 142 and 136 days, respectively, for 7 Fr inside stents. Kaneko et al.11 reported a median time to recurrent biliary obstruction (RBO) of 190 days using 7 to 10 Fr inside stents. A recent RCT12 showed that the median stent patency of 7 Fr inside stents was 123 days. Previous studies reported that the time to RBO for inside stents was 123 to 190 days, longer for inside stents than for PS. The removability of internal stents is an important advantage over uncovered self-expandable metal stents (SEMS). The incidence of RBO is increasing because of the longer survival associated with chemotherapy. Therefore, reintervention may be required for RBO after initial stenting. Inside stent placement for MHBO is advantageous because it can facilitate reintervention in cases of RBO.
Uncovered SEMS
Uncovered SEMS do not obstruct the side branch of the bile duct near the hilar lesion and have a longer stent patency than PS. Two RCTs compared uncovered SEMS and PS in a relatively large population of patients with MHBO.13,14 Mukai et al.13 reported that SEMS are superior to PS in terms of cumulative time to RBO, number of re-interventions, and total cost. The median stent patency times were 359 and 112 days in the SEMS and PS groups, respectively. Sangchan et al.14 also reported that SEMS are superior to PS in terms of clinical success rate, cumulative time to RBO, cumulative survival, and number of re-interventions. The median stent patency was longer for SEMS than PS (103 and 35 days, respectively; p<0.001). Xia et al.15 recently compared bilateral PS placement and bilateral side-by-side (SBS) SEMS placement by using propensity score matching. SBS SEMS placement showed a significantly higher clinical success rate (99.0% and 71.9%, respectively; p<0.001), longer median symptom-free stent patency (9.2 and 4.8 months, respectively; p<0.001), and fewer total interventions (1.3 vs. 2.0; p<0.001) than bilateral PS placement. According to two RCTs13,14 and a propensity score matching analysis,15 uncovered SEMS are superior to PS in terms of functional success, time to RBO, and number of re-interventions. Therefore, uncovered SEMS are recommended for MHBO. However, a lack of removability in the case of RBO is a significant disadvantage of uncovered SEMS, particularly considering the increased survival duration of patients due to recent developments in anti-tumor therapy.
Covered SEMS
Covered SEMS (CSEMS) were developed to prevent tumor ingrowth and have the advantage of easy removability, particularly during reintervention for RBO. Therefore, CSEMS are commonly used to treat malignant distal biliary obstructions. However, CSEMS are not commonly placed in MHBO because they may occlude the side branches of intrahepatic bile ducts. CSEMS with a diameter of 6 mm can be placed for MHBO because their relatively small diameter reduces the risk of intrahepatic bile duct obstruction.16-18 Three retrospective studies evaluated the use of 6-mm-diameter CSEMS for MHBO (Table 1).16-18 In our previous study, the median time to RBO was 210 days for bilateral drainage, and the removal of a fully CSEMS (FCSEMS) was successful in all patients.16 Yoshida et al.17 reported a median stent patency of 95 days and successful stent removal in all patients (7/7) with FCSEMS and 66.7% (4/6) of those with partial CSEMS (PCSEMS). Kitamura et al.18 revealed that the median time to RBO was 79 days and that endoscopic stent removal was successful in six (46%) of 13 patients without tumor ingrowth into the uncovered distal part of the stent. Among the three previous studies, the median time to RBO was longer in our study (210 days) than in the other two studies (95 and 79 days, respectively). SEMS were placed above the papilla in our study and across the papilla in two other studies. The distal end location of the SEMS differed among the studies, which may have affected the time to RBO. Therefore, the placement of a 6-mm CSEMS above the papilla might be advantageous in terms of time to RBO. Furthermore, FCSEMS were easily removed during re-interventions in all cases, whereas PCSEMS could not be removed in all cases. A significant advantage of CSEMS is their removability during reintervention, and FCSEMS are more easily removed than PCSEMS during reintervention.
DRAINAGE AREA
Liver volume and drainage effectiveness
Sufficient liver drainage is required for MHBO. Vienne et al.19 evaluated the association between liver volume on computed tomography and drainage effectiveness and found that the most important factor was drainage of ≥50% of the liver volume. Drainage involving ≥50% of the total liver volume was also associated with longer survival than drainage involving <50% of the total liver volume (119 and 59 days, respectively; p=0.005). Takahashi et al.20 also evaluated the association between the drained liver volume and drainage effectiveness in patients with preserved versus impaired liver function. They revealed that liver volume drainage of ≥33% in patients with preserved liver function and ≥50% in those with impaired liver function promoted good clinical outcomes for MHBO. However, drainage of the atrophic liver lobe should be avoided due to the risk of cholangitis.
Unilateral versus bilateral drainage
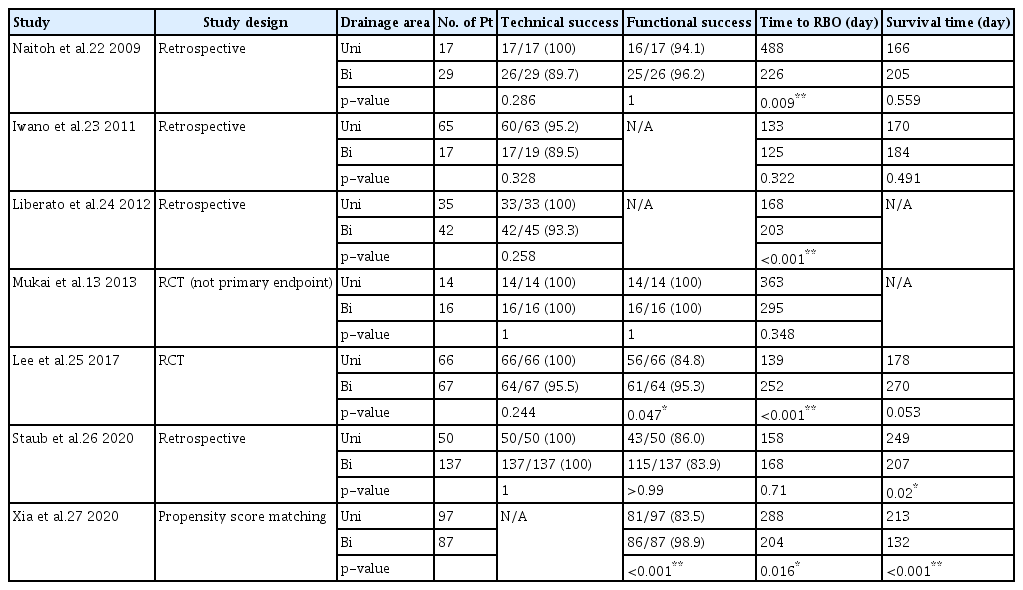
Hilar drainage can be unilateral or bilateral depending on the drainage area. Bilateral drainage is superior because of the greater liver drainage volume. However, endoscopic bilateral drainage is technically difficult compared with unilateral drainage, which is associated with a significantly higher technical success rate and fewer adverse events than bilateral drainage.21-24
Only two RCTs compared unilateral and bilateral drainage as the primary endpoint in MHBO.21,25 De Palma et al.21 conducted an RCT that compared 79 cases of unilateral drainage and 78 cases of bilateral drainage with PS. Unilateral drainage showed a significantly higher technical success rate than bilateral drainage (88.6% and 76.9%, respectively; p=0.041). Bilateral drainage showed a significantly higher complication rate than unilateral drainage (26.9% and 18.9%, respectively; p=0.026). The median survival time did not differ between the unilateral and bilateral drainage groups. Therefore, the authors concluded that unilateral drainage is a safe and feasible strategy. However, a major limitation of the study was the use of PS, considering that SEMS are more commonly used for unresectable MHBO due to their longer stent patency. Lee et al.25 conducted an RCT involving 66 unilateral and 67 bilateral drainage cases by using uncovered SEMS. The clinical success rate was significantly higher in the bilateral drainage group than that in the unilateral drainage group (95.3% and 84.9%, respectively; p=0.047). Furthermore, the cumulative stent patency duration was significantly longer in the bilateral drainage group than in the unilateral drainage group (log-rank test, p<0.01). Interestingly, bilateral drainage was positively associated with survival on the multivariate analysis (95% confidence interval, 0.259–0.666; p<0.01).
Table 2 summarizes the seven previous comparative studies of unilateral and bilateral drainage using SEMS.13,22-27 Bilateral drainage showed a significantly higher functional success rate in two of five studies25,27 in terms of a longer time to RBO in four out of seven studies22,24,25,27 and longer survival time in two of five studies.27 Bilateral drainage was more effective, with a longer time to RBO and longer survival, particularly with SEMS use, in seven comparative studies, including RCTs,13,22-27
BILATERAL DEPLOYMENT METHOD
SBS deployment
In SBS deployment, two SEMS are placed parallel to the common bile duct. The distal ends of the two SEMS are usually located above, rather than across, the papilla. However, no previous study has compared SBS placement above and across the papilla. In sequential SBS, the delivery system of the second SEMS was inserted after the deployment of the first SEMS. However, the recent development of a thin delivery system (<6 Fr) allows the simultaneous insertion and deployment of two SEMS through a single accessory channel of a therapeutic duodenoscope. This new method allows simultaneous SBS placement.28-31 We compared clinical outcomes between sequential and simultaneous SBS deployment31; the technical success rate was significantly higher in the latter case (100% and 71%, respectively; p=0.045). The median procedure time was significantly shorter with simultaneous versus sequential SBS deployment (22 and 52 minutes, respectively; p=0.017). Simultaneous SBS deployment is the optimal technique for bilateral hilar SEMS placement because of its higher success rate and shorter procedure time than sequential SBS deployment. Other studies also reported higher success rates and shorter procedure times with simultaneous versus sequential SBS deployment.29,30,32
In a previous study, we retrospectively evaluated the outcomes of endoscopic reintervention after SBS placement.33 In the multivariate analysis, a narrow common bile duct was a significant predictor of failure of endoscopic reintervention after SBS. The receiver operating characteristic curve showed that a cutoff common bile duct diameter of 7.1 mm predicted technically successful endoscopic reintervention. We recommend the stent-in-stent (SIS) method for patients with a common bile duct diameter <7 mm to promote successful endoscopic reintervention after SBS.
SIS deployment
In SIS deployment, the two SEMS overlap in the common bile duct. However, the mesh of the first SEMS makes it technically difficult to insert the guidewire and delivery system of the second SEMS into the contralateral bile duct. The technical success rate of SIS is increasing owing to the development of new SEMS with a central wide-open mesh stent,34 large cell mesh stent,35 and thin delivery systems. However, there is concern that the use of a large-cell SEMS might lead to a high RBO rate due to tumor ingrowth, although the large-cell SEMS facilitates insertion of the contralateral SEMS in SIS. Lee et al.36 reported that clinical outcomes did not differ significantly between small- and large-cell stents in terms of technical or functional success rate, adverse events, time to RBO, or overall survival. The SIS method commonly uses 8- or 10-mm-diameter SEMS. In a previous study, we retrospectively compared 8- and 10-mm-diameter SEMS in SIS and found that the rates of technical success, functional success, adverse events, and RBO did not differ between them.37 However, the success rate of endoscopic bilateral revisionary stent insertion for RBO was significantly higher in the 10- than 8-mm-diameter group (68% and 31%, respectively; p=0.044). We conclude that the 10-mm-diameter SEMS is more suitable for SIS than the 8-mm-diameter SEMS, particularly with regard to endoscopic reintervention.
Okuno et al.38 retrospectively evaluated the outcomes of endoscopic reintervention after SBS placement and found that a large angle (>104°) between the bilateral SEMS predicted failure of endoscopic reintervention after SIS placement. Therefore, the SBS method may preferable in patients with a large angle between the bilateral SEMS.
SBS versus SIS deployment
Only one RCT compared SBS and SIS SEMS placement for bilateral drainage of MHBO.39 Lee et al.39 conducted an RCT of 35 SBS and 34 SIS SEMS placements. There was no significant difference between SBS and SIS in terms of the rates of total adverse events, technical and clinical success, stent patency, or survival. Table 3 summarizes the five comparative studies of SBS and SIS SEMS placement.29,39-42 The time to RBO did not significantly differ between SBS and SIS in four out of five studies,29,39,40,42 including an RCT, and there were no significant differences between SBS and SIS in terms of the technical success rate, functional success rate, incidence of adverse events other than RBO, time to RBO, or survival time.29,39-42 The diameter of the common bile duct and the angle between the right and left bile ducts could inform the choice of deployment method (i.e., SBS or SIS) considering the risk of endoscopic reintervention failure.33,38
OPTIMAL DRAINAGE STRATEGY
Figure 1 presents the algorithm used to determine optimal endoscopic drainage for MHBO. PS was used for patients with an expected survival duration of at least 3 months. Unilateral stenting was performed in patients who achieved drainage of ≥50% of the total liver volume with a single stent. In contrast, bilateral stenting is performed in patients with <50% drained liver volume. Inside stents are a suitable option for these patients because of their prolonged stent patency. Uncovered SEMS were placed in patients with an estimated survival duration of ≥3 months. An inside stent is suitable for these patients because of their easy removability in cases of reintervention for RBO. In Bismuth II patients, bilateral stenting is recommended because the atrophic lobe is not visible. CSEMS can be placed in these patients. In Bismuth III–IV patients, unilateral stenting of the non-atrophic lobe is recommended for patients with an atrophic lobe, and bilateral or multistenting is recommended when there is no atrophic lobe. The deployment method for bilateral stenting was selected based on the endoscopist’s preference because clinical outcomes did not differ between SBS and SIS. For SBS deployment, simultaneous deployment is recommended because of the shorter procedure time and higher technical success rate than sequential deployment.
CONCLUSIONS
Herein, we reviewed the EBD strategies for unresectable MHBO to determine the optimal strategy. There is no consensus on the optimal stent type, drainage area, or deployment method for optimal drainage. Recent advances in chemotherapy and devices for biliary drainage have improved MHBO treatment. The optimal drainage strategy should be determined in light of new developments including EUS-guided biliary drainage. Additional multicenter prospective studies are required to determine the optimal treatment strategy for unresectable MHBO.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: IN, TI; Data curation: IN, TI; Investigation: IN, TI; Methodology: IN, TI; Visualization: IN, TI; Writing–original draft: IN, TI; Writing–review and editing: IN.