AbstractBackground/AimsAlthough a small amount of fecal material can obscure significant colorectal lesions, it has not been well documented whether bowel preparation status affects the missing risk of colorectal polyps and adenomas during a colonoscopy.
MethodsWe prospectively enrolled patients with one to nine colorectal polyps and at least one adenoma of >5 mm in size at the screening colonoscopy. Tandem colonoscopy with polypectomy was carried out within 3 months.
ResultsA total of 277 patients with 942 polyps and 714 adenomas completed index and tandem examinations. At the index colonoscopy, 187 polyps (19.9%) and 127 adenomas (17.8%) were missed. The per-patient miss rate of polyps and adenomas increased significantly as the bowel cleansing rate declined from excellent to poor/inadequate on the Aronchick scale (polyps, p=0.024; adenomas, p=0.040). The patients with poor/inadequate bowel preparation were independently associated with an increased risk of having missed polyps (odds ratio [OR], 3.21; 95% confidence interval [CI], 1.13 to 9.15) or missed adenomas (OR, 3.04; 95% CI, 1.04 to 8.88) compared to the patients with excellent bowel preparation.
INTRODUCTIONMost of colorectal cancers arise from pre-existing adenomatous polyps.1 Such an adenoma carcinoma sequence provides an opportunity for prevention of colorectal cancers.1-3 However, about 3% to 6% of colorectal cancers are diagnosed between screening and post-screening surveillance examinations,4-10 and the majority of these interval cancers are thought to originate from missed lesions that were overlooked at the screening colonoscopy.11,12 According to emerging evidences, the effectiveness of colonoscopy depend on the quality of the examination.13-15 High quality bowel cleaning is an essential prerequisite to improve the quality of colonoscopy, because even a small amount of residual fecal matter can obscure a significant colorectal lesion. However, suboptimal bowel preparation has been reported as much as 20% of all colonoscopic examinations.16,17 Poor preparation can result not only in prolonged cecal intubation time and withdrawal time, it can also reduce detection rate of both small and large polyps.13 In practice, although guidelines advocate a repeated colonoscopy when suboptimal bowel preparation is detected,11,18,19 the shortening of the interval to the next colonoscopy is often recommended without supporting evidences when confronted.20 To assess the relevance of such an approach, it is necessary to investigate the risk of missing polyps, adenomas and advanced adenomas during the screening colonoscopy depending on the bowel preparation status.21
We performed this prospective study to investigate the risk of missing polyps and adenomas according to the bowel preparation status during colonoscopies using a tandem colonoscopic evaluation.
MATERIALS AND METHODSStudy populationThis study was performed on a consecutive series of patients who had one to nine colorectal polyps and at least one adenoma sized more than 5 mm at the high-quality screening colonoscopy from May 2009 to September 2010 at Konkuk University Medical Center in Seoul, Republic of Korea. The enrolled patients underwent tandem colonoscopy with polypectomy within the next 3 months. The study protocol was approved by the institutional review board of the Konkuk University Medical Center.
Subjects were excluded if they met one of the following criteria: 1) the colonoscopy did not reach the cecum, 2) withdrawal time of index colonoscopy was less than 6 minutes, 3) patients with 10 or more polyps detected at the index colonoscopy and suspected of having polyposis syndrome, 4) tandem colonoscopy was performed 3 months after the index colonoscopy, 5) the bowel preparation at the tandem colonoscopy was fair, poor or inadequate based on the Aronchick scale, 6) patients with a history of bowel resection, and 7) patients with inflammatory bowel disease.
Assessment of the bowel preparation statusThe bowel preparation status was assessed using previously published and validated bowel preparation scales: the Aronchick scale22 and the Ottawa bowel preparation quality scale (Ottawa scale).23 The Aronchick scale assesses the preparation quality of the entire colon as excellent (a small volume of clear liquid or greater than 95% of the surface seen), good (a large volume of clear liquid covering 5% to 25% of the surface but greater than 90% of the surface was seen), fair (some semisolid stool that could be suctioned or washed away, but greater than 90% of the surface was seen), poor (semisolid stool that could not be suctioned or washed away and less than 90% of the surface was seen), or inadequate (repeat preparation and colonoscopy was needed). The Ottawa scale assesses the cleanliness of the right (from the cecum to the ascending colon), mid (from the transverse colon to the descending colon), and recto-sigmoid colon individually by rating each colon segment on a scale of 0 to 4. The fluid quantity is a global value for the entire colon and this is rated from 0 to 2. The score of the Ottawa scale is calculated by adding the cleanliness scores and the fluid quantity score. Thus, the scale has a range from 0 (perfect) to 14 (solid stool in each colon segment and lots of fluid, i.e., a completely unprepared colon). Bowel preparation quality was scored after sufficient washing and suctioning of fecal debris.
Before applying the bowel preparation scales in this study, the participating endoscopists undertook a calibration exercise for achieving excellent inter-observer agreement (intraclass correlation coefficient, ICC >0.8). The calibration exercise was carried out using 10 testing colonoscopy images. If the ICC of the inter-observer agreement failed to reach 0.8, then a calibration exercise with discussions among the endoscopists was repeated. After 2 weeks of calibration exercises, the inter-observer agreement was re-measured using 10 different testing colonoscopy images. This calibration exercise was repeated until excellent inter-observer agreement was achieved among the endoscopists.
ColonoscopyFour experienced endoscopists accredited by the Korean Society of Gastrointestinal Endoscopy participated in this study. The adenoma detection rates of the endoscopists ranged from 34% to 28%. Bowel cleansing was performed using polyethylene glycol (Colyte 4 L; Taejun Pharm. Co., Ltd., Seoul, Korea) or NaP (Fleet; Unimed Pharm. Inc., Seoul, Korea) as previously described.24 For all the study procedures, high-definition CF-H260AI colonoscope (Olympus, Tokyo, Japan) was used. During the index colonoscopy, the participating endoscopists recorded the adenoma characteristics including size, number, shape, and location, withdrawal time, and bowel preparation status assessed by the Aronchick and Ottawa scales. Tandem colonoscopy was performed within 3 months after the index colonoscopy. The median interval between the colonoscopies was 38 days (range, 5 to 89). Suboptimal bowel preparation at screening colonoscopy was associated with several causes, including the failure to follow preparation instructions, later start time of colonoscopy, and history of constipation. To improve the bowel preparation status at the tandem examination, we tried to identify whether or not the patients had consumed the preparation as prescribed. If a participant did not follow the preparation instructions, we strongly recommended him/her to follow the instructions. For participants who followed the preparation instruction, we recommended a longer period of dietary restriction to clear liquids or addition of bisacodyl for those with constipation in order to improve the bowel preparation status at the tandem colonoscopy.
All detected lesions were removed during the tandem colonoscopy using snare polypectomy or endoscopic mucosal resection. In order to find and remove the colorectal adenomas during the tandem colonoscopy, the size, location, and shape of the detected lesions were recorded in the data sheet in detail during the index colonoscopy. Since polyps of Ōēż5 mm is often impossible to find at tandem colonoscopy, they were removed during the index colonoscopy using cold biopsy polypectomy or cold snare polypectomy.
The adenoma size was estimated during the index colonoscopy using open-biopsy forceps. Adenoma were categorized as diminutive (Ōēż5 mm), small (5 to 9 mm) or large (Ōēź10 mm) according to their size. The location of the adenoma was classified as right (from cecum to ascending colon), mid (from transverse colon to descending colon), or recto-sigmoid colon as described in the Ottawa scale.23 The shape of a colorectal adenoma was classified as pedunculated, sessile, or flat/depressed. A flat/depressed lesion was defined as an endoscopically visible flat and/or depressed mucosal lesion with a height less than half the diameter of the lesion.25,26
StatisticsContinuous variables are expressed as the mean┬▒standard deviation, while categorical variables are presented as absolute values and percentages. Differences between the continuous variables were analyzed using the unpaired Student's t-test, and differences between the categorical variables were analyzed using the Žć2 test and Fisher's exact test as appropriate.
To assess the inter-observer agreement, the ICC and 95% predictive interval (PI) for the Aronchick and Ottawa scales were calculated. An ICC greater than 0.80 is defined as excellent agreement.27 The statistical correlation between the Aronchick and Ottawa scales was calculated using Spearman's rank correlation test.
The relationship between the bowel preparation status assessed by the Aronchick scale and the missed polyps, adenomas, or advanced adenomas was analyzed using the Žć2 test for trends. The per-patient miss rate was calculated by dividing the number of patients with missing lesions at the index colonoscopy by the total number of patients.28 The per-polyp miss rate was calculated by dividing the number of missing lesions at the index colonoscopy by the total number of lesions found either on the index or tandem colonoscopies.28
To investigate the risk of missing polyps, adenomas or advanced adenomas in a patient according to the bowel preparation status assessed by Aronchick scale, multivariate analysis was performed using logistic regression analysis adjusted with age, gender, withdrawal time, and number of polyps detected at index colonoscopy. For each variable, the odds ratio (OR) and 95% confidence interval (CI) were reported. A p-value of less than 0.05 was considered to indicate statistical significance. The analyses were performed with SPSS software version 12.0K (SPSS Inc., Chicago, IL, USA).
RESULTSAssessment of the inter-observer agreement for the bowel preparation statusAt 2 weeks after the initial calibration exercise, the inter-observer agreement for assessing the bowel preparation status was first measured using the ICCs for the Aronchick and Ottawa scales, which were 0.749 (95% PI, 0.495 to 0.919) and 0.862 (95% PI, 0.690 to 0.958), respectively. After the second calibration exercise, the ICCs for the Aronchick and Ottawa scales reached 0.822 (95% PI, 0.615 to 0.945) and 0.880 (95% PI, 0.724 to 0.964), respectively.
Per-patient analysisA total of 277 patients with 942 polyps and 714 adenomas completed the 1st and tandem colonoscopies with polyp removal within 3 months. Table 1 shows the clinical characteristics of the study patients. The mean age was 56.2┬▒11.3 years, and 191 patients were male. There were no significant differences in gender or number of polyps and adenomas detected at the index colonoscopy between the patients with and without missed polyps, adenomas or advanced adenomas. However, the patients with missed adenomas were older than those without a missed advanced adenoma. There was a strong positive correlation between the Aronchick and Ottawa scales (r=0.917; p<0.001).
The bowel preparation of the index colonoscopy, according to the Aronchick scale, was described as excellent in 88 patients (32%), good in 114 patients (41%), fair in 56 patients (20%), poor in 17 patients (6%), and inadequate in two patients (1%). Table 2 shows the per-patient miss rate analysis of polyp, adenoma, and advanced adenoma according to bowel preparation status. When the bowel preparation status was assessed by the Aronchick scale, the per-patient miss rate of polyps, adenomas and advanced adenomas increased significantly as bowel preparation declined from excellent, to good, to fair, and to poor/inadequate (per-patient polyp miss rate, 27%, 35%, 36%, and 58%, respectively, p=0.024; per-patient adenoma miss rate, 21%, 27%, 27%, and 47%, respectively, p=0.040; per-patient advanced adenoma miss rate, 9%, 17%, 18%, and 37%, respectively, p=0.006). In addition, when the bowel preparation was assessed by the Ottawa scale, the score was higher in patients with missed lesions, compared to those without a missed lesion (polyp, 3.4┬▒2.4 vs. 4.2┬▒3.2, p=0.036; adenoma, 3.5┬▒2.5 vs. 4.3┬▒3.2, p=0.046; advanced adenoma, 3.5┬▒2.6 vs. 4.6┬▒3.2, p=0.015) (Table 3).
To identify whether the bowel preparation status is an independent variable associated with missed polyps, adenomas or advanced adenomas, multivariate analyses were performed (Table 4). The patients with poor/inadequate bowel preparation were independently associated with an increased risk of having missed polyps (OR, 3.21; 95% CI, 1.13 to 9.15), missed adenomas (OR, 3.04; 95% CI, 1.04 to 8.88), or missed advanced adenomas (OR, 5.28; 95% CI, 1.58 to 17.68) compared to those with excellent bowel preparation.
Per-polyp analysisAmong the total 942 polyps, 187 polyps were missed at the screening colonoscopy, with 19.9% of polyp miss rate. Among the total 714 adenomas, 127 adenomas were missed at the screening colonoscopy, producing 17.8% of adenoma miss rate. When the bowel preparation was excellent, good, fair, or poor/inadequate, the miss rate of polyps, adenomas, and advanced adenomas significantly increased (polyp miss rate, 14%, 17%, 26%, and 48%, respectively, p<0.001; adenoma miss rate, 12%, 17%, 22%, and 40%, respectively, p<0.001; advanced adenoma miss rate, 9%, 6%, 19%, and 58%, respectively, p<0.001) (Table 5).
DISCUSSIONEven though colonoscopy is considered the "Criterion Standard" for the detection of colorectal neoplasms,19,29,30 colonoscopies are not infallible. Previous tandem colonoscopy studies have reported that the miss rate for overall adenomas ranged from 12% to 24%.28,31-34 Interestingly, these tandem colonoscopy studies demonstrated miss rates of colonoscopy for adenomas Ōēź1 cm in size between 0% and 6%,28,31-34 which have been increased recently to 12% to 17% due to results from computed tomography colonography studies.35-37 However, these studies enrolled only patients with an adequate bowel preparation.35-37 It is not a special occasion tosee a patient with suboptimal bowel preparation in daily colonoscopy practice.16,17 The patients with suboptimal bowel preparation are thought to be associated with increased risk of having missed colorectal neoplasms, but there has been no report on the risk of missing polyps and adenomas during screening colonoscopy according to the bowel preparation status.
Instead, most of previous studies have evaluated the effect of bowel preparation on adenoma detection rate.10,15,17,38 A larger retrospective study using approximately 93,000 colonoscopies recorded in the Clinical Outcome Research Initiative identified higher detection rates in the cases with adequate preparation versus those with inadequate preparation (26% vs. 29%, p<0.001).17 This finding was supported by another study of 5,832 patients, which reported that the detection of neoplasms, including polyps of any size as well as large lesions (Ōēź10 mm), were associated with the quality of bowel preparation.38 However, the most reliable method to evaluate the quality of colonoscopy is considered to assess the risk of missing lesion during colonoscopy by performing tandem colonoscopy.21
In this study, when the bowel preparation was assessed by the most commonly used validated bowel preparation scales, the Aronchick scales, the per-patient miss rate and per-polyp miss rate of polyps, adenomas, and advanced adenomas increased significantly as the bowel preparation became suboptimal. Interestingly, the per-patient miss rates for polyps, adenomas, and advanced adenomas increased remarkably between fair and poor/inadequate preparations (polyp, 36% to 58%; adenoma, 27% to 47%; advanced adenoma, 18% to 37%). In addition, when the bowel preparation was assessed by the Ottawa scale, mean Ottawa scores were higher in the patients with missed lesions than the patients without a missed lesion (polyp, 3.4┬▒2.4 vs. 4.2┬▒3.2, p=0.036; and adenoma, 3.5┬▒2.5 vs. 4.3┬▒3.2, p=0.046). Multivariate analyses also revealed that the patients with poor/inadequate bowel preparation status was associated with an increased risk of a missed polyp, missed adenoma, and missed advanced adenoma. In contrast to previous studies, our results showed that the withdrawal time was not associated with the risk of missed lesions. One possible reason is that the cases with less than 6 minutes of withdrawal time for the index colonoscopy were excluded in our study.
The practical guidelines for bowel preparation status endorse only one of two options: a repeat colonoscopy upon inadequate preparation or colonoscopies at regular intervals for satisfactory preparation.11,18,19 However, up to 20% of patients who have had a colonoscopy were reported as suboptimal,17,38 and repeated colonoscopies can induce high medical cost and unexpected complications. When confronted with an intermediate-quality or low-quality preparation, most endoscopists recommend a shorter follow-up interval, rather than repeating the procedure.20 This variability and uncertainty may be related to the fact that the incremental yield of repeating a colonoscopy after suboptimal bowel preparation is not known. Our study documented that the patients with poor/inadequate bowel preparation have increased risk of having missed polyps (OR, 3.21; 95% CI, 1.13 to 9.15), missed adenomas (OR, 3.04; 95% CI, 1.04 to 8.88), and missed advanced adenomas (OR, 5.28; 95% CI, 1.58 to 17.68) compared to those with excellent bowel preparation. Therefore, it might be recommended that the interval of follow-up colonoscopy should be reduced for patients with suboptimal bowel preparation, as most endoscopists already do.
Our study has several limitations. First, when determining the true polyp miss rate of colonoscopy, the patients with adenomas as well as those without adenomas were included. The finding of adenomas at index colonoscopy increases the chance of having missed adenomas.32 All participants in our study had one or more adenoma larger than 5 mm. Therefore, it is likely that the overall miss rate was inflated in this study and did not imply the real polyp miss rate in clinical practice.28,31-34 Second, we excluded patients with more than 10 polyps, because patients with 10 or more adenomatous polyps are at increased probability of having missed lesions.18 Although the patients with numerous polyps were not common, the exclusion of patients with numerous polyps could introduce bias. In addition, Among the 277 enrolled patients, 187 patients (67.5%) underwent tandem colonoscopy with polypectomy by the same colonoscopist who performed their screening colonoscopy. Remaining patients underwent tandem colonoscopy by different colonoscopists in this study. Different adenoma detection rates and techniques between the colonoscopists could lead to bias.
In conclusion, it is clear that suboptimal bowel preparation not only prolongs the overall procedure time,38 decreases the cecal intubation rates,38,39 leads to increased costs associated with colonoscopy,40 it also increases the risk of missing polyps or adenomas during the colonoscopy. The colonoscopy procedure rarely missed advanced adenomas when the bowel preparation was adequate, whereas the risk of missing advanced adenomas increased significantly when the bowel preparation was poor or inadequate. Therefore, repeated examination or shortening the colonoscopy follow-up interval might be suitable strategies for a patient with suboptimal bowel preparation. Future large-scaled multi-center studies to evaluate polyp and adenoma miss rate according to bowel preparation status in average-risk patients undergoing screening colonoscopy will be needed to further stratify the risk of developing interval cancer, as well as to determine the cost-effectiveness of repeated examination versus shortening of the colonoscopy follow-up interval in patients with suboptimal bowel preparation.
AcknowledgmentsThis work was supported by the Konkuk University Medical Center Research Grant 2009.
References1. Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525ŌĆō532. 2841597.
2. Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329:1977ŌĆō1981. 8247072.
3. Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut 2001;48:812ŌĆō815. 11358901.
4. Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006;4:1259ŌĆō1264. 16996804.
5. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 2007;132:96ŌĆō102. 17241863.
6. Hosokawa O, Shirasaki S, Kaizaki Y, Hayashi H, Douden K, Hattori M. Invasive colorectal cancer detected up to 3 years after a colonoscopy negative for cancer. Endoscopy 2003;35:506ŌĆō510. 12783349.
7. Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 2005;129:34ŌĆō41. 16012932.
8. Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest Endosc 2010;71:111ŌĆō117. 19647250.
9. Gorski TF, Rosen L, Riether R, Stasik J, Khubchandani I. Colorectal cancer after surveillance colonoscopy: false-negative examination or fast growth? Dis Colon Rectum 1999;42:877ŌĆō880. 10411433.
10. Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy 2004;36:499ŌĆō503. 15202045.
11. Bond JH. Should the quality of preparation impact postcolonoscopy follow-up recommendations? Am J Gastroenterol 2007;102:2686ŌĆō2687. 18042104.
12. Pabby A, Schoen RE, Weissfeld JL, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary polyp prevention trial. Gastrointest Endosc 2005;61:385ŌĆō391. 15758908.
13. Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol 2006;101:873ŌĆō885. 16635231.
14. Thomas-Gibson S, Rogers P, Cooper S, et al. Judgement of the quality of bowel preparation at screening flexible sigmoidoscopy is associated with variability in adenoma detection rates. Endoscopy 2006;38:456ŌĆō460. 16767579.
15. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362:1795ŌĆō1803. 20463339.
16. Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci 2010;55:2014ŌĆō2020. 20082217.
17. Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003;58:76ŌĆō79. 12838225.
18. Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872ŌĆō1885. 16697750.
19. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570ŌĆō1595. 18384785.
20. Ben-Horin S, Bar-Meir S, Avidan B. The impact of colon cleanliness assessment on endoscopists' recommendations for follow-up colonoscopy. Am J Gastroenterol 2007;102:2680ŌĆō2685. 17714555.
21. van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006;101:343ŌĆō350. 16454841.
22. Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc 2000;52:346ŌĆō352. 10968848.
23. Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc 2004;59:482ŌĆō486. 15044882.
24. Seol DC, Hong SN, Kim JH, et al. Change in renal function after sodium phosphate preparation for screening colonoscopy. World J Gastroenterol 2010;16:2010ŌĆō2016. 20419839.
25. Soetikno R, Friedland S, Kaltenbach T, Chayama K, Tanaka S. Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology 2006;130:566ŌĆō576. 16472608.
26. Aaltonen LA, Hamilton SR. World Health Organization, International Agency for Research on Cancer. Pathology and Genetics of Tumours of the Digestive System. 2000. 2nd ed. Lyon: IARC Press.
27. Muller R, B├╝ttner P. A critical discussion of intraclass correlation coefficients. Stat Med 1994;13:2465ŌĆō2476. 7701147.
28. Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;40:284ŌĆō290. 18389446.
29. Niv Y, Hazazi R, Levi Z, Fraser G. Screening colonoscopy for colorectal cancer in asymptomatic people: a meta-analysis. Dig Dis Sci 2008;53:3049ŌĆō3054. 18463980.
30. Marbet UA, Bauerfeind P, Brunner J, Dorta G, Valloton JJ, Delco F. Colonoscopy is the preferred colorectal cancer screening method in a population-based program. Endoscopy 2008;40:650ŌĆō655. 18609465.
31. Hixson LJ, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990;82:1769ŌĆō1772. 2231773.
32. Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24ŌĆō28. 8978338.
33. Bensen S, Mott LA, Dain B, Rothstein R, Baron J. Polyp Prevention Study Group. The colonoscopic miss rate and true one-year recurrence of colorectal neoplastic polyps. Am J Gastroenterol 1999;94:194ŌĆō199. 9934755.
34. Kasugai K, Miyata M, Hashimoto T, et al. Assessment of miss and incidence rates of neoplastic polyps at colonoscopy. Dig Endosc 2005;17:44ŌĆō49.
35. Van Gelder RE, Nio CY, Florie J, et al. Computed tomographic colonography compared with colonoscopy in patients at increased risk for colorectal cancer. Gastroenterology 2004;127:41ŌĆō48. 15236170.
36. Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: key concepts regarding polyp prevalence, size, histology, morphology, and natural history. AJR Am J Roentgenol 2009;193:40ŌĆō46. 19542393.
37. Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007;357:1403ŌĆō1412. 17914041.
38. Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc 2005;61:378ŌĆō384. 15758907.
Table┬Ā2Per-Patient Miss Rate of Polyp, Adenoma, and Advanced Adenoma According to Bowel Preparation Assessed by Aronchick Scale 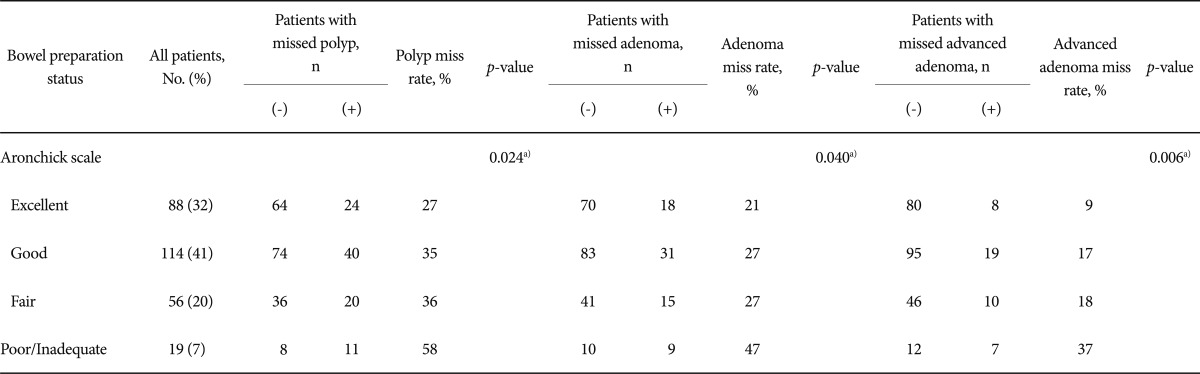
Table┬Ā3Per-Patient Miss Rate of Polyp, Adenoma, and Advanced Adenoma According to Bowel Preparation Assessed by Ottawa Scores 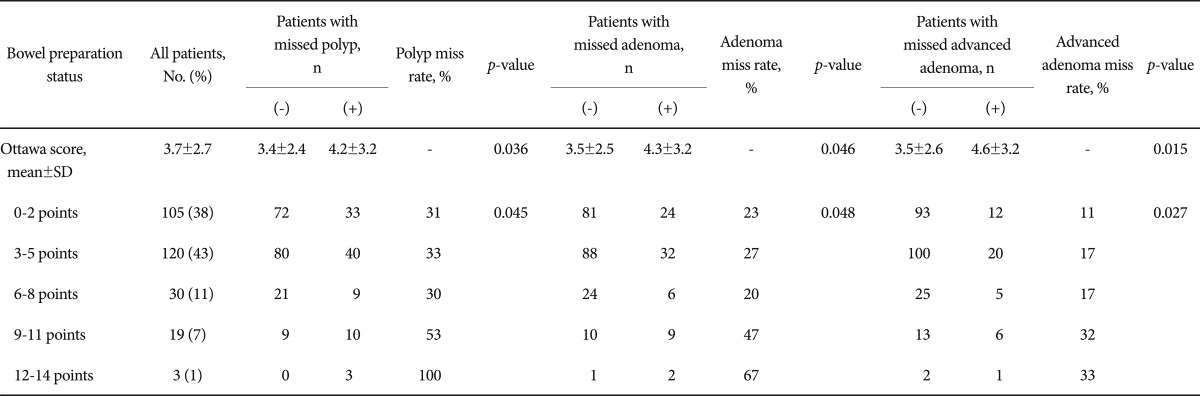
|
|
||||||||||||||||||||||||||||||||||||||
