INTRODUCTION
Estimation of invasion depth is one of the most important steps for establishing therapeutic plans for colorectal tumors. Without deep submucosal invasion, lymph node metastasis rarely occurs in colorectal tumors,1-3 and those tumors can be treated with local excision such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD).
The depth of invasion of colorectal tumors can be estimated by using the gross findings of conventional white light endoscopy (CWE), pit patterns identified by magnifying chromoendoscopy (MCE),4,5 and the surface microvascular patterns identified by magnifying narrow band imaging endoscopy (MNE).6-8 However, their accuracy and interobserver agreement were rarely reported.
Thus, this study was performed to evaluate the accuracy and interobserver agreement of expert endoscopists for predicting the depth of invasion using CWE, MCE, and MNE.
MATERIALS AND METHODS
Subjects
All of the CWE, MCE, and MNE electronic photos and video clips of the cases were recorded with magnifying colonoscopes (CF-H260AZL; Olympus Co., Tokyo, Japan) by an expert endoscopist (B.I.L) from November 2009 to October 2010. Thirty-three colorectal tumors were included and all the tumors had more than one of gross features suggesting early colorectal cancer (Tis or T1); which are hardness, surrounding white spots, depression, surface nodularity, full expansion, deformed adjacent wall, mucosal friability, convergence of mucosal folds, erosion, or ulceration.9,10 Grossly obvious advanced cancers with luminal obstruction or large ulceration were excluded.
All of the photos and video clips were taken from as many angles as possible after washing the lesions with simethicone-dissolved water. The CWE was recorded first, followed by MNE and MCE. Observation with magnifying endoscopy was performed more carefully for depressed or erosive areas. MCE was performed with 0.4% indigo carmine and 0.05% crystal violet in sequence. When crystal violet was applied, observation was not made until the dye had sufficiently stained the tumor.
Estimation of invasion depth
Three expert endoscopists (S.W.K, H.C, and K.Y.C) independently reviewed the electronic photos and video clips of the cases. The endoscopists had experiences of more than 10,000 colonoscopy cases and had used magnifying endoscopy for several years.
Kudo's classification was used for pit pattern analysis.12 Microvascular patterns were categorized according to the Showa Classification.7,13 Unlike pit pattern, classifications for microvascular pattern had not been standardized at the time of the study,14 and the Showa Classification was therefore reviewed by the endoscopists before the estimation of invasion depth.
Only the information on sex and age of the patients was provided to the endoscopists. The endoscopists reviewed the CWE, the MCE, and the MNE images in sequence.
Based on the CWE, the endoscopists evaluated gross findings suggestive of submucosal invasion (depression, full expansion, wall deformity, spontaneous bleeding, mucosal convergence, erosion, or ulcer)5,15 and the depth of invasion (mucosa vs. submucosa or beyond). The estimations were made again based on the MCE, and then on the MNE. A "VN pattern" on the MCE12 and a "sparse pattern" on the MNE7,13 were considered suggestive of submucosal invasion (Fig. 1).
The study protocol was approved by the Institutional Review Board (OC11RIMI0011).
Statistical analysis
The SAS system version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. Differences of diagnostic accuracy for submucosal invasion among the endoscopists and among the methods of endoscopy were evaluated by Generalized Estimating Equations. The p-values were corrected by Bonferroni's method. The interobserver agreement among the endoscopists was evaluated by Fleiss' kappa.
RESULTS
Baseline characteristics
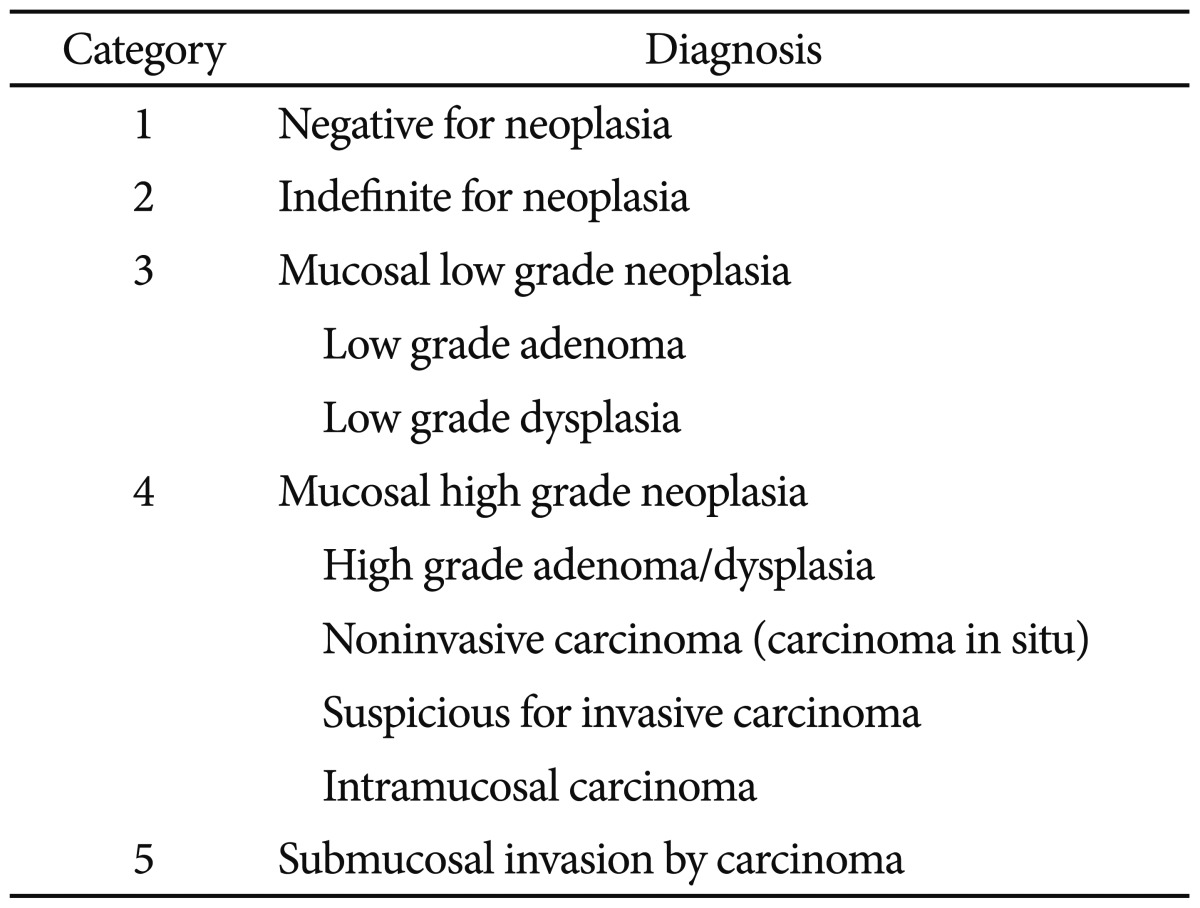
The median diameter of the lesions was 24 mm (range, 10 to 61). The lesions included 13 protruded type, one flat type, two depressed type, and 17 laterally spreading tumors. The tumors were treated primarily by EMR (11), ESD (17), and surgery (5). One of the patients who had undergone EMR received additional surgery later due to invasion to the deep submucosal layer. The histopathologic results were as follows: mucosal low-grade neoplasia (low grade adenoma/dysplasia) in nine patients; mucosal high grade neoplasia (high grade adenoma/dysplasia, noninvasive carcinoma, and intramucosal carcinoma) in 16 patients; and submucosal or further invasion by carcinoma) in eight patients.
Diagnostic accuracy for submucosal invasion
Among the endoscopists, the diagnostic accuracy for submucosal invasion using gross findings of CWE ranged from 67% to 82%, while those using pit patterns identified by MCE and microvasculature patterns identified by MNE ranged from 85% to 88% and 85% to 88%, respectively (Fig. 2).
There was no significant difference in diagnostic accuracy among the endoscopists (p=0.257). However, the diagnostic accuracy significantly differed among the methods of endoscopy (p=0.040). The interaction between the endoscopists and the methods of endoscopy was not significant (p=0.086).
The diagnostic accuracy significantly differed between CWE and MCE (p=0.034, Bonferroni's correction) and between CWE and MNE (p=0.039, Bonferroni's correction). However, the diagnostic accuracy did not significantly differ between MCE and MNE (p>0.05).
DISCUSSION
"Early colorectal cancer" is defined by Japanese doctors as colorectal cancer limited to the mucosa or invading only the submucosa, regardless of the presence or absence of lymph node metastases.16 However, in this study, we evaluated the diagnostic accuracy of submucosal invasion for "early colorectal cancer-like lesions" instead of "pathologically confirmed early colorectal cancers." Only the gross features suggesting early colorectal cancer9,10 were used for the selection criteria.
The evaluation of early colorectal cancer-like lesions can be more practical than the evaluation of confirmed early colorectal cancers; this is because, first, it is not always easy to achieve endoscopic differentiate between a carcinoma and an adenoma or even between early cancer and advanced cancer. In other words, when an early colorectal cancer-like tumor is detected during a colonoscopy, we cannot confirm whether the lesion is an early colorectal cancer until the final pathology report of the resected specimen has been obtained. The role of forceps biopsy is also limited since the histopathology of endoscopic forceps biopsy disagrees with that of a resected specimen in 40% of cases.17
Second, when establishing therapeutic plans, it may be more important to determine whether the tumor is limited to the mucosal layer rather than whether the tumor is a carcinoma or an adenoma. Most mucosal neoplasms (revised Vienna classification categories 3 and 4:11 low grade adenoma/dysplasia, high grade adenoma/dysplasia, noninvasive carcinoma, and intramucosal carcinoma) can be treated by local excision (EMR or ESD) because lymph node metastasis is rarely reported without deep submucosal invasion.
The diagnostic accuracy for submucosal invasion was significantly improved by pit pattern analysis with MCE in this study. Moreover, the interobserver agreement of MCE was substantial, while the interobserver agreement of CWE was moderate. As such, MCE can estimate the depth of invasion for early colorectal cancer-like lesions more accurately and more objectively.
The diagnostic accuracy for submucosal invasion also significantly differed between CWE and MNE. However, the diagnostic accuracy did not differ between MCE and MNE. The estimation of submucosal invasion using MNE might be limited since the irregular microvascular pattern by the Showa classification could be interpreted in different ways. We considered an "irregular microvascular pattern" identified using MNE as a mucosal cancer without submucosal invasion. However, according to other studies,13,14 more than half of colorectal tumors with an irregular microvascular pattern showed deep submucosal invasion. In addition, microvascular classifications of colorectal tumors based on MNE had not been unified at the time of the study,14 and all of the expert endoscopists in this study were more familiar with pit pattern analysis using MCE than microvascular pattern analysis using MNE.
It is possible that the results would have been different if the endoscopists viewed the MNE images before the MCE images. In practice, MNE is performed before MCE because the applied dye used for chromoscopy interferes with MNE. However, in this study, estimation was performed earlier by MCE than by MNE because we set out to identify any additional effects of MNE on MCE for predicting submucosal invasion.
After the depth of invasion was estimated, we examined the cases in which the results of MCE and MNE did not agree among the endoscopists. The reasons for disagreement were mixed pit/microvascular patterns and bad images due to bleeding, exudation, or insufficient staining.
In conclusion, the estimation of submucosal invasion based on MCE or MNE is more accurate and more helpful for establishing a treatment strategy for colorectal tumors than the estimation based on CWE. MCE and MNE were demonstrated to have substantial agreement for estimating the depth of invasion.