INTRODUCTION
Subepithelial masses are a common endoscopic finding and are currently being encountered at an increasing rate given the widespread use of gastrointestinal (GI) endoscopy. The term subepithelial refers to a mass, bulge, or impression visible from the lumen arising from any layer of the GI wall or an adjacent structure causing extrinsic compression. The prevalence on routine endoscopy is unknown; however, one retrospective study reported subepithelial gastric lesions in 0.36% of esophagogastroduodenoscopies performed between 1976 and 1984.1 The differential diagnosis for these lesions is broad and includes benign, premalignant, malignant, and normal structures (Table 1). Treatment strategies vary accordingly, thus definitive diagnosis remains the utmost priority.
ENDOSONOGRAPHY
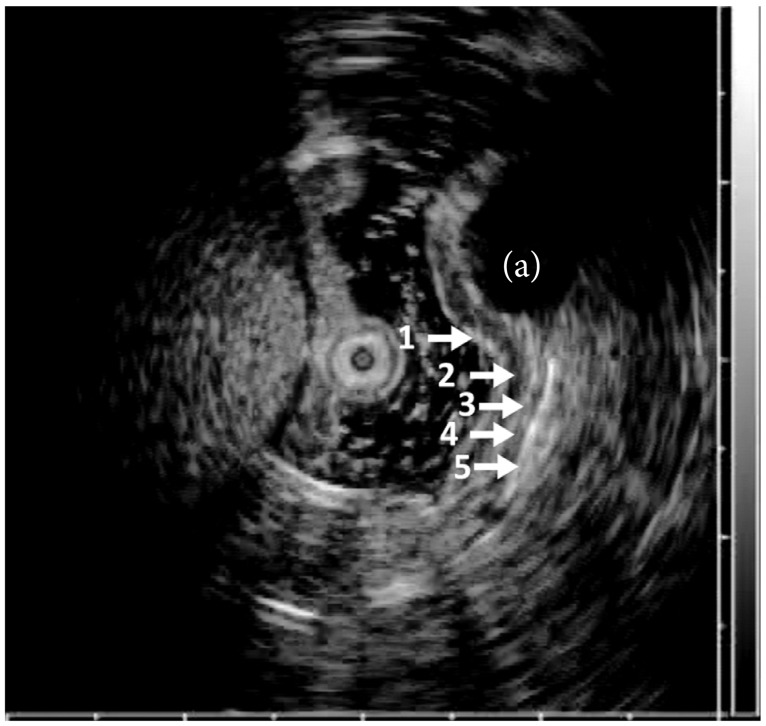
Endoscopic ultrasound (EUS) uses high frequency (5 to 20 MHz) sound waves to obtain detailed images of the GI tract wall and extraluminal structures and reliably differentiates between intramural wall lesions and extrinsic structures. EUS imaging of the GI tract typically identifies five distinct layers (Fig. 1), but can differentiate between seven or nine layers depending on the location of use and frequency of ultrasound probe. These layers have been histologically correlated.2 Characteristics of an intramural lesion including size, layer of origin, echotexture, and margins can be useful adjunctive information for accurate identification. Previous reports suggest extension between layers, irregular margins, or invasion into adjacent structures favor a malignant lesion aiding to guide treatment.3,4 However, endoscopic evaluation alone is insufficient for diagnosing the etiology of a subepithelial lesion.5 EUS has provided a major breakthrough for the characterization of subepithelial lesions, but histology is still needed in most situations.
TISSUE ACQUISITION
Although rarely diagnostic, it is reasonable to perform biopsies of the mucosa overlying the subepithelial lesions.6 Stacked biopsies can be attempted; however, the yield remains low. Bite on bite technique using conventional sized forceps ranging from two to eight bites had a 38% diagnostic rate (54% in the esophagus, 28% in the stomach and duodenum) in a recent study.7 As such, techniques including endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), EUS-guided Trucut biopsy (TCB), and EUS-guided fine needle biopsy (FNB) have been introduced to increase the diagnostic yield.
Pathologists have long preferred as much tissue as possible since the diagnosis typically requires immunohistochemical (IHC) staining for increased diagnostic yield. For subepithelial tumors (SETs), cytology, cell block processing, and IHC staining are all available using a 22-gauge EUS-FNA needle.8 IHC and mitotic counts usually cannot be performed on slides prepared for cytology; however, IHC can often be performed on cell blocks if sufficient quantities of cells have been collected.9 Therefore, a great deal of effort has been put forth to increase the diagnostic yield in subepithelial lesions, but each advancement continues to show its own distinct limitations.
EUS-FNA needles
EUS-FNA using 19-, 22-, or 25-gauge needles are commonly used to obtain tissue from suspicious lesions identified on EUS imaging. FNA with a 22-gauge needle can be used to obtain a cytological specimen with the occasional core tissue specimen by directing the needle into the lesion under ultrasound guidance (Fig. 2A).10 The sensitivity of EUS-FNA cytology for the diagnosis of GI stromal tumor was reported to be 78.4%; however, this sensitivity value was based on the identification of cells with spindle cell morphology and did not require confirmation with IHC.11 In this study, 62% of cases had a definitive diagnosis with IHC and 22% had a diagnosis that was suspicious for gastrointestinal stromal tumor with the finding of spindle cell morphology but not confirmed with IHC. IHC staining of various cell proteins can be performed on FNA samples if a sufficient quantity of cells is obtained to provide additional diagnostic information; however, critical architecture remains absent and a major drawback to FNA as a sole diagnostic procedure.
Recently, the Olympus Prototype Side-Port Needle (Olympus Corp., Tokyo, Japan) was developed in attempt to increase tissue acquisition and reduce the required number of overall passes for the diagnosis. In a pilot study, diagnostic material was obtained at the first pass in 56.2% of patients with the mean number of passes prior to diagnosis of 2.1. Overall, the diagnosis was reached in 94% of the patients. The safety profile was equivalent to similar products, but further prospective randomized trials are needed.12
Trucut core biopsy
To overcome the limitations of FNA, a 19-gauge Trucut core biopsy needle (QuickCore; Wilson-Cook Inc., Winston-Salem, NC, USA) has been proposed.13,14 This needle provides a core of tissue that can not only be used for individual cell morphology, but can be histologically examined for architectural change. Initial experience used in five patients with intramural lesions yielded the correct diagnosis in four of five cases compared to one of five cases using EUS-FNA.13 Some difficulties, such as needle stiffness, misfire of the needle inside the lesion and procedural difficulty when the lesion was in the distal antrum, were reported using EUS-TCB.15 A subsequent retrospective study looked at gastric SET greater than 2 cm in size. Nine were not able to be punctured and 19 more were unable to obtain adequate tissue. However, treatment plans were changed for 18 of 65 patients (27.7%) resulting in avoiding unnecessary resection, scheduling definitive treatment, and modifying the surgical field.16
Combination EUS-FNA with EUS-FNB
Both EUS-FNA and FNB have unique drawbacks limiting diagnostic accuracy. Combining these two techniques has been evaluated retrospectively with an overall accuracy of 95% (76% for EUS-FNA, 76% for EUS-FNB, 95% for combo; p=0.007) without an on site cytopathologist present.15 A prospective study followed demonstrating the yield of adequate tissue harvesting was similar for EUS-FNA and EUS-TCB (96.4% vs. 89.3%; p=not significant), with the same number of passes done. However, the accuracy for obtaining a specific diagnosis was significantly lower for EUS-FNA compared with EUS-TCB (5.3% and 68.4%; p<0.005). The information obtained was complementary, but EUS-TCB was useful when IHC was needed for definitive diagnosis of subepithelial mesenchymal tumors.17 A larger series followed showing EUS-FNA of subepithelial lesions was diagnostic in 61.6% and showed a spindle cell neoplasm (suspicious) in another 22.3% for an overall diagnostic yield of 83.9%. EUS-FNB did not increase the yield in this series.6 A randomized study comparing 22-gauge FNA and FNB needles in solid pancreatic masses further supported equal diagnostic sufficiency, technical performance, and safety profiles between the needles.18 In practice, combining EUS-FNA and EUS-FNB may not be practical due to the increased number of passes and higher cost. Instead, one method might be used as a rescue strategy when another one is failing.
ProCore needle
Due to limitations of the Trucut needle, a new histological needle with a core trap, a 19-gauge EUS-FNB device (ProCore; Cook Endoscopy, Winston-Salem, NC, USA), has been developed.19 This needle is uniquely designed to obtain both cytology and histology using reverse bevel technology, aiming for diagnosis on decreased number of passes. Core tissue samples can often be obtained using the ProCore needle making it possible to perform histology as well as IHC staining to help characterize SET (Fig. 2B). In a recent European study, the diagnostic accuracy was greater than 90% using this new 19-gauge EUS-FNB needle.20 The 22- and 25-gauge needles are also available.21 However, further studies are needed to validate this approach in subepithelial lesions.
COMPLICATION RATES
EUS-FNA and core biopsy has emerged as a safe and effective diagnostic as well as therapeutic modality in recent years. The unique optical and mechanical properties require close attention during this procedure, but small series indicate the use of FNA needles by experienced operators does not carry an increased risk of complications.13,14,22,23 A learning curve exists for FNA, which may have a bearing on complications;24 however, the optimal number of procedure during training has not been defined. Theoretically, FNA or core biopsy of a subepithelial lesion carries a reduced complication rate when compared to transmural tissue acquisition.
CONCLUSIONS
For definitive diagnosis of subepithelial lesions, tissue acquisition from the lesion is necessary with both cytological and histochemical examinations including IHC stains. EUS-FNA is an effective method for obtaining tissue, but remains limited. EUS with Trucut appears to improve diagnostic accuracy, but is also limited by various factors including cumbersome use and variable yield. These two modalities should be considered complementary with the use of Trucut when IHC and tissue architectural details are required for diagnosis. Recently, EUS-guided core biopsy with a ProCore needle has become available. This allows core specimens with more tissue, IHC staining, and architecture, but more studies are needed to determine if the use of this particular needle improves the diagnostic yield in obtaining a tissue diagnosis in subepithelial lesions.