AbstractExtramedullary plasmacytoma involves organs outside the bone marrow; however, involvement of the pancreas is rare. We recently experienced a case of extramedullary plasmacytoma of the pancreas that was diagnosed by endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA). EUS-FNA, which has a high diagnostic accuracy and an excellent safety profile, is the modality of choice for establishing tissue diagnosis. We report a case of extramedullary plasmacytoma of the pancreas diagnosed using EUS-FNA.
INTRODUCTIONPlasma cell neoplasms are characterized by the neoplastic proliferation of a single clone of plasma cells, typically producing a monoclonal immunoglobulin. Plasma cell neoplasms can present as a single lesion (solitary plasmacytoma) or as multiple lesions (multiple myeloma). Solitary plasmacytomas most frequently occur in the bone (plasmacytoma of bone), but can also be found outside the bone in soft tissues (extramedullary plasmacytoma).1 Approximately 5% of all cases of plasma cell disorders are solitary plasmacytomas of the bone.2 However, the involvement of the pancreas is rare. We report a case of an extramedullary plasmacytoma of the pancreas diagnosed using ultrasonography-guided fine needle aspiration (EUS-FNA) with a review of the literature.
CASE REPORTA 58-year-old woman was transferred to our hospital for further evaluation of pelvic pain that was aggravated by walking and began approximately 2 months before admission. The initial laboratory tests showed a hemoglobin level of 10.6 g/dL, and blood urea nitrogen was 12.0 mg/dL. The results of liver function tests were within normal limits. The level of total protein level was 9.7 g/dL and the albumin level was 3.3 g/dL. A peripheral blood cell smear revealed mild lymphopenia. Skeletal surveys detected a small osteolytic lesion without a sclerotic rim in the left parietal bone and a large bone destructive osteolytic lesion in the right inferior pelvic bone (Fig. 1). Incisional biopsy of the right pelvic bone suggested a malignant bone tumor. The lesion was diagnosed as a plasmacytoma. Further evaluation showed a serum free light chain lambda level of 3,475.3 mg/L and a ЮВ2-microglobulin level of 4.6 mg/L. Immunoelectrophoresis showed the presence of an abnormal band of immunoglobulin G and lambda lanes. Plasma cells were 14.2% on bone marrow biopsy. Contrast-enhanced abdominal computed tomography (CT) revealed a suspicious ill-defined marginated mass in the body of the pancreas (Fig. 2). To further characterize the lesion, magnetic resonance imaging (MRI) of the pancreas was performed. T2-weighted MRI indicated that the pancreatic proximal body contained a mass of subtle high signal intensity (Fig. 3A) with marked diffusion restriction (Fig. 3B). For the histopathologic diagnosis, EUS-FNA was planned. Linear EUS (EU-ME1 Ultrasound System; Olympus, Tokyo, Japan) (GF-UCT 240; Olympus) revealed a 1.2У1.0-cm sized, hypoechoic, heterogeneous, well-defined round mass in the pancreatic body (Fig. 4A); FNA was performed via a transgastric approach and five passages were made with a 22-gauge needle (EchoTip Ultra, ECHO-22; Cook Endoscopy, Winston-Salem, NC, USA) (Fig. 4B). The cytopatholgy results showed a small cell neoplasm and the immunohistochemical profile was compatible with plasmacytoma (Fig. 5). The patient began combination therapy consisting of bortezomib, mephalan, and prednisolone with local radiation therapy for the right pelvic bone lesion. The patient was followed up carefully.
DISCUSSIONMultiple myeloma is a disease characterized by malignant proliferation of plasma cells typically involving medullary bones. Myelomatosis, a solitary bone myeloma, or an extramedullary plasmacytoma are possible presentations. Extramedullary plasmacytomas represent 3% to 4% of all plasma cell neoplasms and have a male predominance, with a three to five times higher incidence in men than in women; they usually occur in the sixth and seventh decades of life. Approximately 80% to 90% of tumors develop in the head and neck area, although gastrointestinal involvement has been reported in 10% of cases. Pancreatic involvement of myeloma is relatively rare, with an incidence rate of 2.3% based on autopsy studies. However, previous studies failed to describe the type of disease entity, and did not distinguish between multiple myeloma, myelomatosis, solitary bone myeloma, or extramedullary plasmacytoma.3-6
The radiological differentiation of extramedullary plasmacytoma of the pancreas from other pancreatic tumors such as poorly differentiated pancreatic neoplasm, lymphoma, and metastasis is difficult. Most cases of plasma cell infiltration of the pancreas are microscopic, and well-formed masses are unusual. The latter may present as a focal mass or a diffuse enlargement of the pancreas; typically, the patient presents with jaundice and abdominal pain related to the obstruction of the biliary tree because pancreatic plasmacytoma is often located in the head of the pancreas. In the present case, the mass was detected incidentally because it was located in the body of the pancreas. On ultrasound, pancreatic plasmacytoma appears as a multilobulated heterogenous or a homogenous hypoechoic mass. The CT features have been described as a focal multilobulated solid hypodense mass with homogeneous intravenous contrast enhancement or diffuse enlargement of the pancreas. MRI features include pancreatic enlargement with a lobulated contour, lower signal intensity than that of the liver on T1-weighted images, and diffusely increased signal intensity on T2-weighted pulse sequences with heterogeneous enhancement.7,8 In our case, imaging findings were compatible with those of previous Western reports.
The diagnosis of an extramedullary plasmacytoma is based on evidence of a monoclonal plasma cell tumor outside the bone marrow. In the present case, we performed EUS-FNA of the pancreas. EUS-FNA has an excellent safety profile regarding the risk of pancreatitis, bleeding, and perforation. Major complications have been reported in 2.5% of 355 patients who underwent EUS-FNA for a solid pancreatic mass.9 Potential complications include acute pancreatitis, infection, and sedation-related complications; no deaths have been reported.10 Seeding of the needle track with tumor cells is rare. Previous studies reported that the risk of seeding of pancreatic adenocarcinoma via EUS-FNA is 2.2%, which is low compared with that (16.3%) of CT or transabdominal ultrasonography-guided percutaneous biopsy.11,12 Moreover, seeding of a pancreatic extramedullary plasmacytoma during EUS-FNA is yet to be reported.
In conclusion, we safely performed EUS-FNA in a patient with pancreatic plasmacytoma, and were able to provide an accurate visualization and histopathologic diagnosis. Although there are approximately three case reports describing pancreatic involvement of multiple myeloma diagnosed by EUS-FNA in the English language literature,7,13,14 to the best of our knowledge, this is the first time it is reported in Korea.
References1. Soutar R, Lucraft H, Jackson G, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol 2004;124:717т726. 15009059.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol 2009;144:86т94. 19016727.
3. Miljkovic' M, Senadhi V. Use of endoscopic ultrasound in diagnosing plasmacytoma of the pancreas. JOP 2012;13:26т29. 22233943.
4. Bachar G, Goldstein D, Brown D, et al. Solitary extramedullary plasmacytoma of the head and neck: long-term outcome analysis of 68 cases. Head Neck 2008;30:1012т1019. 18327783.
5. Schabel SI, Rogers CI, Rittenberg GM, Bubanj R. Extramedullary plasmacytoma. Radiology 1978;128:625т628. 674630.
6. Fischer A, Suhrland MJ, Vogl SE. Myeloma of the head of the pancreas. A case report. Cancer 1991;67:681т683. 1985760.
7. Padda MS, Milless T, Adeniran AJ, Mahooti S, Aslanian HR. Pancreatic and gastric plasmacytoma presenting with obstructive jaundice, diagnosed with endoscopic ultrasound-guided fine needle aspiration. Case Rep Gastroenterol 2010;4:410т415. 21060710.
8. Gupta P, Rice GD, Abraham K, Ghole V, Ketkar M. Extramedullary plasmacytoma of the pancreas and jejunum. Clin Imaging 2009;33:240т243. 19411034.
9. Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006;63:622т629. 16564863.
10. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087т1095. 9097990.
11. Katanuma A, Maguchi H, Hashigo S, et al. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy 2012;44(Suppl 2 UCTN):E160тE161. 22622721.
12. Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 2003;58:690т695. 14595302.
Fig.Т 1Pelvic magnetic resonance imaging shows a large bone destructive mass with a soft tissue mass in the right inferior pelvic bone (arrow). 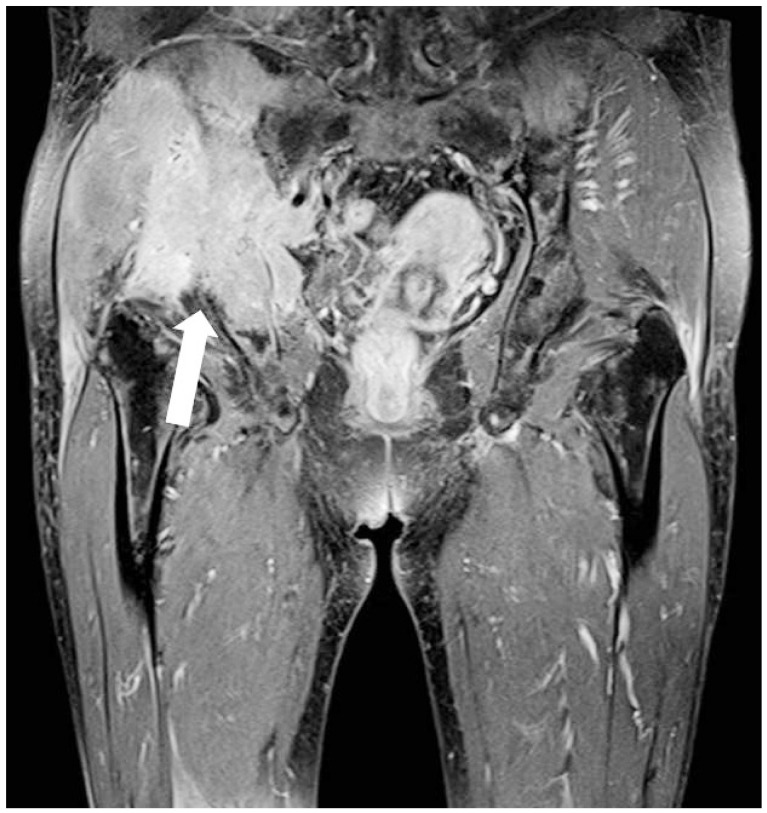
Fig.Т 2Contrast-enhanced abdomen-pelvis computed tomography shows a suspicious ill-defined marginated mass in the body of the pancreas (arrow). 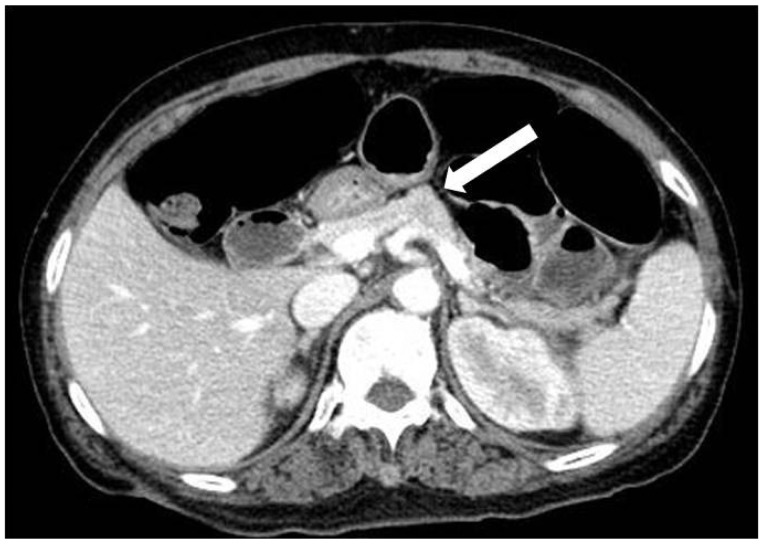
Fig.Т 3T2-weighted magnetic resonance imaging with a diffusion weighted image shows that the pancreatic proximal body contains (A) a mass of subtle high signal intensity with (B) marked diffusion restriction (arrows). 
|
|
||||||||||||||||||||||||||||||||||||||||
