See commentary "Endoscopic Biliary Drainage for Hilar Obstruction: Further Evidence But Still A Long Way To Go" in Volume 54 on page 629 AbstractBackground/AimsMany unanswered questions remain about the treatment of malignant hilar obstruction. We investigated endoscopic stenting for malignant biliary strictures, as reported in a nationwide registry.
MethodsAll endoscopic retrograde cholangiopancreatography (ERCP) procedures entered in the Swedish Registry of Gallstone Surgery and ERCP from January 2010 to December 2017 in which stenting was performed for malignant biliary stricture management were included in this study. Patency was estimated by determining the time to reintervention.
ResultsEndoscopic stenting was performed for malignant stricture management in 4623 ERCP procedures, of which 1364 (29.5%) were performed for hilar strictures. Of the hilar strictures, 320 (23.5%) were intrahepatic strictures (BismuthâCorlette IIIâIV). Adverse events were more common after hilar stenting than after distal stenting (17.2% vs. 12.0%, p<0.0001). The 6-month reintervention rate was 73.4% after hilar stenting compared with 55.9% after distal stenting (p<0.0001). The 6-month reintervention rates for BismuthâCorlette types I, II, IIIa, IIIb, and IV were 70.4%, 75.6%, 90.0%, 87.5%, and 85.7%, respectively. In multivariate analysis, the risk for reintervention was three times higher after hilar stenting than after distal stenting (hazard ratio 3.47, 95% confidence interval 2.01â6.00, p<0.001).
INTRODUCTIONAlthough multiple issues about the management and outcomes of malignant biliary strictures have been addressed in a number of study formats, few studies to date have compared the outcomes of endoscopic stenting specifically according to different locations in the biliary tree. A number of randomized trials have shown the superiority of self-expanding metal stents (SEMSs) over plastic stents for both distal strictures (DSs) and hilar strictures (HSs) [1,2]. The equivalence of covered and uncovered SEMSs for DSs has been confirmed in numerous studies [3]. In contrast, although hilar stenting is inherently more complex and generates more questions, fewer studies with generally lower levels of evidence and weaker treatment recommendations are available concerning the management of HSs. More data from unselected populations are necessary to assess the outcome of stenting as practiced in the community at large.
In this respect, a number of controversies and unanswered questions remain about the management of malignant HSs. The percentage of liver volume that needs to be drained to achieve symptom control in advanced BismuthâCorlette (BâC) types is a topic of ongoing debates, with evidence supporting both 30% (unilateral stenting) and 50% (requiring multiple or bilateral stenting) [4â7]. Studies with relatively small patient cohorts are complicated by multiple possible confounders. These include variations in the complexity and definition of strictures (level, extent, and multiplicity), access used for stent placement (transhepatic vs. transpapillary), and different stent configurations (stent-in-stent vs. side-by-side) in patients with BâC type IIIâIV strictures.
Therefore, we designed this study in which the aforementioned confounders were minimized to answer a specific research question based on prospective data from a well-validated and large population-based registry. The purpose of this study was to assess endoscopic stenting for malignant biliary obstruction according to stricture location (distal vs. hilar, further subdivided into the different hilar BâC types). The primary endpoints were procedural and 30-day adverse events, and the secondary endpoint was the need for reintervention.
MATERIALS AND METHODSStudy designThis study conformed to the provisions of the Declaration of Helsinki and was approved by the Stockholm Regional Research Ethics Committee (EPN no. 2018/277-31). Since May 2005, endoscopic retrograde cholangiopancreatography (ERCP) procedures performed in Sweden have been entered into the Swedish Registry of Gallstone Surgery and ERCP (GallRiks, http:/www.ucr.uu.se/gallriks). This nationwide registry, covering >90% of all hospitals in Sweden, has confirmed high validity and follow-up frequency, offering reliable information on indications, prevailing ERCP practice, nature and technical success of interventions, and adverse events [8,9].
All ERCP procedures performed for malignant biliary obstruction and entered into GallRiks between January 1, 2010, and December 31, 2017, were assessed for inclusion in the study (Fig. 1). During this period, endobiliary radiofrequency ablation has not yet been introduced in Sweden. Only index procedures performed for malignant strictures in which the diagnosis was confirmed on cytology/histology or at a multidisciplinary team discussion (based on tumor markers and imaging) were included. Patients in whom the International Classification of Diseases 10th revision coding did not confirm malignancy (bile duct stones [K80] and indeterminate strictures [K83]) were excluded. Likewise, patients who did not receive a stent because of unsuccessful cannulation or an inability to cross the stricture with a guidewire (complete stenosis) were excluded. Patients in whom the stricture or stent position was not indicated (missing data) were further excluded. Therefore, only patients with malignant biliary obstruction with known positions of both the stricture/s and stent/s were included in the analysis of adverse events. To calculate the stent failure rate, patients in whom a plastic stent or a combination of a plastic stent and a SEMS was placed, as well as patients in whom multiple SEMSs were applied at the index ERCP were excluded, such that only patients who received a single SEMS were analyzed.
The analyzed data included sex, age, and American Society of Anesthesiologists (ASA) physical status classification. Procedural information included the anatomical location of the stricture (divided into DS and HS, with a further subdivision into extrahepatic HS [BâC IâII] and intrahepatic HS [BâC IIIâIV]) and the total procedure time. Both intraprocedural and 30-day postprocedural adverse events were documented. In patients who required endoscopic reintervention, the time to reintervention was compared between DSs and HSs, as well as among HSs subdivided into different BâC types. All patients were followed up until December 31, 2018. The date of death in deceased patients was determined by cross-referencing with the Swedish Central Death Register.
DefinitionsThe index procedure was defined as the first ERCP procedure performed. The most proximal stricture extension was used as a reference point for defining the stricture location. A DS was defined as an obstruction situated either in the ampulla of Vater or the common bile duct (ductus choledochus), distal to the cystic duct junction. An HS was defined as an obstruction involving or situated proximal to the cystic duct junction in the common hepatic duct (ductus hepaticus) and/or left and/or right hepatic ducts, and HSs were subdivided according to the modified BâC classification [10]. The total procedure time was defined as the time from endoscope insertion to the time of scope removal.
Adverse events were defined according to internationally accepted consensus criteria, in which pancreatitis is defined as abdominal pain in the presence of an increase in serum amylase levels to at least three times the normal level and cholangitis is defined as a new onset temperature >38°C at >24 h after the procedure combined with cholestasis [11]. The timing of adverse events was classified as intraprocedural or postprocedural (â€30 days). The time to the need for endoscopic reintervention (owing to symptoms associated with recurrent obstruction) was used as a proxy for stent failure or patency regardless of cause (occlusion or migration), according to the Tokyo criteria for reporting on transpapillary biliary stenting [12].
Statistical analysisAll statistical analyses were performed using JMP Pro 14.2.0 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were used for demographic and clinical parameters, procedure time, and adverse events, with Pearsonâs chi-square test used for categorical data and Studentâs t-test for continuous data. Stent failure and stent patency were calculated using the KaplanâMeier method, and Cox proportional hazard analysis was used to assess the influence of sex, age, ASA score, and stricture location on the risk of reintervention. Endoscopic reintervention was considered a terminal event, whereas death or reaching the end of the study period with a functioning stent was treated as a censored event.
RESULTSOf 58,981 ERCP procedures registered during the inclusion period, 4623 fulfilled the inclusion criteria (Fig. 1). Of the patients, 70.5% had a DS (n=3,259) and 29.5% had an HS (n=1,364), with 76.5% of the HS cases being BâC type I or II (Fig. 2). The demographic parameters, ASA category, procedure time, and adverse events in patients stented for a DS or an HS are presented in Table 1. Patients undergoing hilar stenting had a mean age of 71.4 years (p=0.0054), and 55% of them were women (p=0.0201). Patients stented for HS more often had an ASA III or IV status (53.2% vs. 40.5%, p<0.0001) and a longer mean procedure time (51.9 vs. 36.9 min, p<0.0001). Postprocedural (30-day) adverse events were significantly more common after hilar stenting (17.2% vs. 12.0%, p<0.0001), with pancreatitis occurring in 6.6% of patients stented for HSs compared with 4.0% of patients stented for DSs (p=0.0001). The incidence of postprocedural cholangitis was higher after hilar stenting than after distal stenting (4.1% vs. 2.8%, p=0.0239).
When specific BâC types were compared, patients with intrahepatic strictures (BâC IIIâIV) were younger (mean age 68.8 vs. 72.3 years, p<0.0001) and had a longer mean procedure time (65.9 vs. 47.6 min, p<0.001) than patients with extrahepatic strictures (BâC IâII) (Table 2). Both intraprocedural and 30-day postprocedural adverse events did not significantly differ between extrahepatic and intrahepatic strictures (2.2% vs. 1.6% [p=0.4796] and 17.7% vs. 15.3%, [p=0.3175]).
Fig. 3 shows the time to the need for reintervention after stent placement for hilar versus distal malignant obstruction. Reintervention within 6 months after stent placement was performed in 73.4% of the patients stented for HSs compared with 55.9% of patients stented for DSs (p<0.0001). At 12 months, the percentage of patients who had not required reintervention was 11.2% in the hilar stenting group and 22.9% in the distal stenting group (p<0.0001). Reintervention within 6 months after stent placement was performed in 70.4%, 75.6%, 90.0%, 87.5%, and 85.7% of BâC I, II, IIIa, IIIb, and IV cases, respectively (Fig. 4). In multivariate analysis taking into account sex, age, and ASA category, male sex and HS location were identified as significant risk factors for reintervention. A three times higher risk of reintervention was observed in patients with an HS (hazard ratio 3.47, 95% confidence interval 2.01â6.00, p<0.001) than in patients with a DS (Table 3).
DISCUSSIONOur study, based on prospective data from a large and well-validated registry, is, to our knowledge, the first to directly compare the outcomes of endoscopic stenting for malignant strictures located in the distal and hilar (subdivided into different BâC types) locations of the biliary tree. Although the incidence of cholangiocarcinoma is increasing in Western countries, the distribution of malignant strictures between DSs (70%) and HSs (30%) in our study reflects the prevalence of pancreatic adenocarcinoma, cholangiocarcinoma, and gallbladder cancer found in populations worldwide [13].
The superiority of SEMSs over plastic stents for malignant DSs and HSs and the equivalence of covered and uncovered SEMSs for DSs have been confirmed in multiple randomized trials [1â3]. Whereas both covered and uncovered SEMSs could be used for BâC I HSs, probably with comparable results, uncovered SEMSs are used for BâC IIâIV strictures to prevent the occlusion of biliary radicles covered by the expanded stent proximal to the stricture [14]. The recommendations for HS management are generally weak, as they are based on low-level evidence. Studies are typically hampered by a multitude of variables and potential confounders, including the proximal and distal extent of strictures, liver functionality (presence of atrophied segments and number of segments drained), diverse underlying pathologies (cholangiocarcinoma vs. metastatic strictures), and access type (percutaneous vs. ERCP vs. endoscopic ultrasound). The presence of these multitude of variables results in small numbers for analysis, and statistically significant results are seldom reported.
The mean procedure time was shorter for DSs than for HSs and was similar between extrahepatic and intrahepatic HSs. These results were expected, as incrementally more proximal strictures could be technically more challenging to traverse with a guidewire owing to decreased thrust and increased angulation encountered in the hilum. The difficulty in selectively cannulating specific intrahepatic ducts for bilateral or multisectoral stenting also adds to the procedure time. Postprocedural adverse events, specifically post-ERCP pancreatitis and cholangitis, more commonly occurred after hilar stenting than after distal stenting. It is known that the risk of post-ERCP pancreatitis is not influenced by performing an endoscopic sphincterotomy before SEMS deployment but is directly related to the procedure time, and the higher rates in our study may be related to the significantly longer procedure times [15]. The cholangitis rates after distal endoscopic stenting for malignant obstruction vary depending on stent type (SEMS vs. plastic stent), ranging from 5% to 30% in randomized controlled trials [16,17]. Prospective studies on hilar endoscopic stenting for malignant obstruction have reported cholangitis rates ranging between 4.7% and 16.6% [18â21]. Cholangitis after hilar stenting is multifactorial and can be the result of procedural factors, such as inadvertent filling of bile ducts not drained by the placed stent, and tumor progression. Interestingly, our data did not show significant differences in 30-day adverse events (including cholangitis) when comparing intrahepatic and extrahepatic HS stenting. Although the definitions of cholangitis vary, our results are in contrast with those of previous publications reporting cholangitis rates for BâC I, II, and IIIâIV of 4%, 10%, and 57.7%, respectively, and those reporting that BâC IV stricture stenting was associated with higher cholangitis rates [22,23].
The mean patency duration after distal SEMS placement has been reported to be 250 days, and randomized trials report 6- and 12-month patency rates varying between 68%â78% and 32%â55% [19,24]. Our study found an estimated 6-month patency rate of 44%, with 56% of the patients requiring reintervention. For hilar stents, patency rates of 30% and 17% at 6 and 12 months, respectively, have been reported in randomized controlled trials, although most reports failed to investigate differences among the BâC types [18,25]. Importantly, our data showed estimated 6- and 12-month patency rates for hilar stents of 27% and 11%, respectively, with reintervention required in 73% at 6 months and in 89% at 12 months. In multivariate analysis, male sex and HS location were identified as risk factors for reintervention, with the risk of reintervention after HS stenting being three times higher than that after DS stenting.
In a retrospective report on 61 patients undergoing hilar stenting, Rerknimitr et al. reported mean patency durations of 87, 69, and 41 days for BâC types I, II, and IIIâIV respectively [22]. The 6-month reintervention rates in the current study ranged from 70% for BâC I strictures to 86% for BâC IV strictures. This is likely the effect of proximal disease progression occluding initially drained segments that were colonized, resulting in cholangitis and requiring additional interventions. Although the numbers were small, the highest reintervention rate at 6 months was observed in SEMSs placed in the right hepatic duct (90%), in contrast to previous reports of decreased patency in left-sided SEMSs, but highlighting the fact that bilateral or multisectoral drainage should probably be pursued in more advanced BâC types [26]. Placement of a single stent in patients with a BâC I stricture allows for drainage of the whole liver, comparable to single stent placement in DSs. It is interesting to note the 14% difference in patency rates after DS (44%) and BâC I stricture (30%) stenting in our patients.
To our knowledge, this is the largest analysis of hilar stent performance as it occurs in the population at large. However, the study has a number of limitations. Hilar strictures were defined as obstructions involving or proximal to the opening of the cystic duct, which, owing to variation in the level of the cystic duct junction, may have resulted in an overlap between distal and BâC I strictures in terms of actual stricture position. Although there is still no consensus on an alternative definition, the use of a measured cutoff, such as the distal half of the extrahepatic bile duct for DSs, as proposed by the Japanese Society of Hepato-Biliary-Pancreatic Surgery, would be more appropriate and would probably generate more clinically relevant data [27]. As prophylactic nonsteroidal anti-inflammatory and antibiotic use was only introduced as part of GallRiks data capturing in the latter period of participant inclusion, these confounders could not be accounted for. Death without reintervention was treated as a censored event; however, the actual stent patency at the time of death could not be determined, and random censoring and an occluded stent at the time of death could not be excluded. Percutaneous reintervention for stent dysfunction and patients undergoing curative resection or palliative chemotherapy could not be accounted for, as nonendoscopic interventions are not entered into the registry. Not accounting for these groups probably underestimated the number of stent failures, especially for intrahepatic HSs.
This study underscores the difficulties encountered in investigating stenting for malignant HSs. The study simplified and did not cover the whole spectrum of hilar stenting, as patients treated with plastic or multiple stents and percutaneous or endoscopic ultrasound-placed stents were excluded. Randomized trials that have generated strong evidence about the numerous issues concerning DS stenting cannot be replicated for HS stenting. Analyses of large population-based cohorts could serve as a platform for studies to obtain more robust data. However, simple ERCP registries such as GallRiks, not specifically designed for HSs, lack granular information on key variables, thus precluding in-depth analyses of important unanswered questions.
In conclusion, our study, based on a large and well-validated population-based registry, confirms that endoscopic treatment of malignant HS has high complication rates and high reintervention rates. The number of variables that need to be accounted for in this population precludes comprehensive randomized trials. Dedicated multi-institutional registries specifically designed to answer research questions about hilar stenting are needed.
NOTESFunding
This study was supported by the South African Medical Research Council through the Self-Initiated Research Grant.
Author Contributions
Conceptualization: Urban Arnelo, Lars Enochsson
Data curation: Jeanne Lubbe, UA, Eduard Jonas, LE
Formal analysis: JL, Gabriel Sandblom, EJ, LE
Funding acquisition: JL
Investigation: JL, GS, UA, EJ
Methodology: JL, GS, EJ, LE
Project administration: LE
Resources: GS, UA, LE
Software: GS, LE
Supervision: LE
Validation
Visualization
Writing-original draft: JL
Writing-review&editing: JL, GS, UA,EJ, LE
Fig. 1.Flowchart of the included ERCP procedures in this study. ERCP, endoscopic retrograde cholangiopancreatography; ICD, International Classification of Diseases. 
Fig. 3.Time to the need for endoscopic reintervention (in months) after hilar and distal single metal stent placement. SEMS, self-expanding metal stent (log-rank: chi-square [ChiSq] 47.07, p>ChiSq<0.0001; Wilcoxon: ChiSq 66.13, p>ChiSq<0.0001). 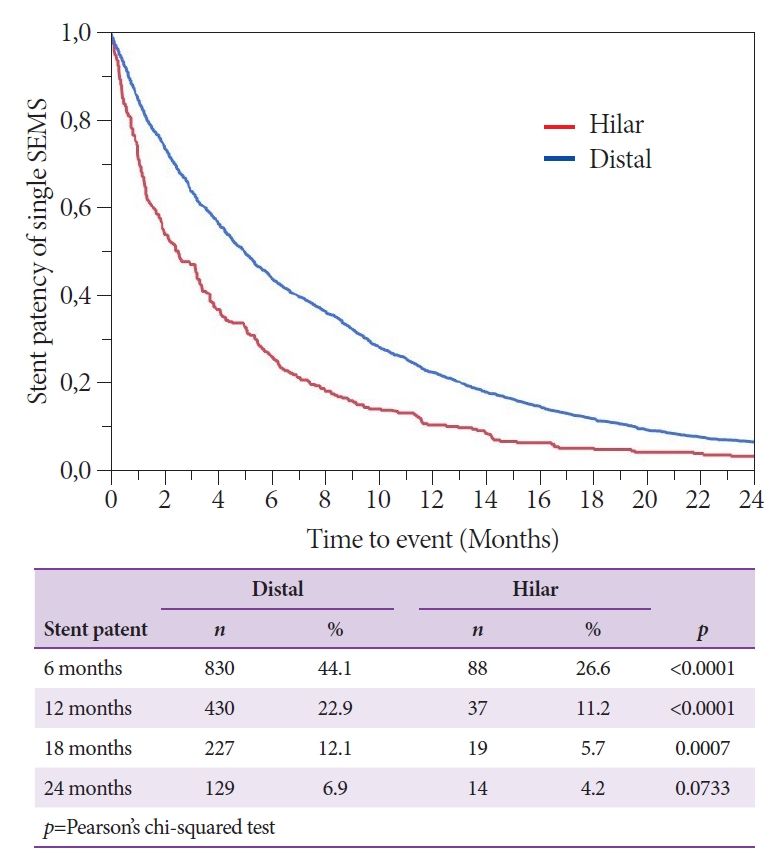
Fig. 4.Time to the need for endoscopic reintervention (in months) after single metal stent placement for BâC types IâIV. SEMS, self-expanding metal stent; BâC, BismuthâCorlette (log-rank: chi-square [ChiSq] 58.85, p>ChiSq<0.0001; Wilcoxon: ChiSq 74.04, p>ChiSq< 0.0001). 
Table 1.Demographic Details, Procedural Information, and Adverse Events Comparing Endoscopic Transpapillary Stenting for Distal versus Hilar Strictures
Table 2.Demographic Details, Procedural Information, and Adverse Events Comparing Endoscopic Transpapillary Sstenting for Extrahepatic Hilar Strictures (BâC IâII) versus Intrahepatic Hilar Strictures (BâC IIIâIV)
Table 3.Univariate and Multivariate analyses of the Risk for Reintervention after Single Metal Stent Placement
REFERENCES1. Almadi MA, Barkun A, Martel M. Plastic vs. self-expandable metal stents for palliation in malignant biliary obstruction: a series of meta-analyses. Am J Gastroenterol 2017;112:260â273.
2. Hong W, Chen X, Wu W-Z, Zhu Q, Chen X. Metal versus plastic stents for malignant biliary obstruction: an update meta-analysis. Clin Res Hepatol Gastroenterol 2013;37:496â500.
3. Tringali A, Hassan C, Rota M, Rossi M, Mutignani M, Aabakken L. Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: a systematic review and meta-analysis. Endoscopy 2018;50:631â641.
4. Baer HU, Rhyner M, Stain SC, et al. The effect of communication between the right and left liver on the outcome of surgical drainage for jaundice due to malignant obstruction at the hilus of the liver. HPB Surg 1994;8:27â31.
5. Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc 2010;72:728â735.
6. Ashat M, Arora S, Klair JS, Childs CA, Murali AR, Johlin FC. Bilateral vs unilateral placement of metal stents for inoperable high-grade hilar biliary strictures: a systemic review and meta-analysis. World J Gastroenterol 2019;25:5210â5219.
7. Aghaie Meybodi M, Shakoor D, Nanavati J, et al. Unilateral versus bilateral endoscopic stenting in patients with unresectable malignant hilar obstruction: a systematic review and meta-analysis. Endosc Int Open 2020;8:E281âE290.
8. Rystedt J, Montgomery A, Persson G. Completeness and correctness of cholecystectomy data in a national register--GallRiks. Scand J Surg 2014;103:237â244.
9. Enochsson L, Blohm M, Sandblom G, et al. Inversed relationship between completeness of follow-up and coverage of postoperative complications in gallstone surgery and ERCP: a potential source of bias in patient registers. BMJ Open 2018;8:e019551.
10. Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31â38.
11. Dumonceau J-M, Kapral C, Aabakken L, et al. ERCP-related adverse events: european society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 2020;52:127â149.
12. Isayama H, Hamada T, Yasuda I, et al. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc 2015;27:259â264.
13. Tyson GL, Ilyas JA, Duan Z, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci 2014;59:3103â3110.
14. Dumonceau J-M, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results: european society of gastrointestinal endoscopy (ESGE) clinical guideline - updated october 2017. Endoscopy 2018;50:910â930.
15. Sofi AA, Nawras A, Alaradi OH, Alastal Y, Khan MA, Lee WM. Does endoscopic sphincterotomy reduce the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis after biliary stenting? A systematic review and meta-analysis. Dig Endosc 2016;28:394â404.
16. Moses PL, Alnaamani KM, Barkun AN, et al. Randomized trial in malignant biliary obstruction: plastic vs partially covered metal stents. World J Gastroenterol 2013;19:8638â8646.
17. Schmidt A, Riecken B, Rische S, et al. Wing-shaped plastic stents vs. self-expandable metal stents for palliative drainage of malignant distal biliary obstruction: a randomized multicenter study. Endoscopy 2015;47:430â436.
18. Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc 2012;76:93â99.
19. ZorrĂłn Pu L, de Moura EGH, Bernardo WM, Baracat FI, et al. Endoscopic stenting for inoperable malignant biliary obstruction: a systematic review and meta-analysis. World J Gastroenterol 2015;21:13374â13385.
20. De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc 2001;53:547â553.
21. Lee TH, Kim TH, Moon JH, et al. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: a multicenter, prospective, randomized study (with video). Gastrointest Endosc 2017;86:817â827.
22. Rerknimitr R, Kladcharoen N, Mahachai V, Kullavanijaya P. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J Clin Gastroenterol 2004;38:518â523.
23. Xia M-X, Wang S-P, Wu J, et al. The risk of acute cholangitis after endoscopic stenting for malignant hilar strictures: a large comprehensive study. J Gastroenterol Hepatol 2020;35:1150â1157.
24. Conio M, Mangiavillano B, Caruso A, et al. Covered versus uncovered self-expandable metal stent for palliation of primary malignant extrahepatic biliary strictures: a randomized multicenter study. Gastrointest Endosc 2018;88:283â291.e3.
25. Mukai T, Yasuda I, Nakashima M, et al. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci 2013;20:214â222.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
