AbstractComputer-assisted polyp characterization (computer-aided diagnosis, CADx) facilitates optical diagnosis during colonoscopy. Several studies have demonstrated high sensitivity and specificity of CADx tools in identifying neoplastic changes in colorectal polyps. To implement CADx tools in colonoscopy, there is a need to confirm whether these tools satisfy the threshold levels that are required to introduce optical diagnosis strategies such as ŌĆ£diagnose-and-leave,ŌĆØ ŌĆ£resect-and-discardŌĆØ or ŌĆ£DISCARD-lite.ŌĆØ In this article, we review the available data from prospective trials regarding the effect of multiple CADx tools and discuss whether they meet these thresholds.
INTRODUCTIONColorectal cancer (CRC) is the third most common cancer worldwide and a major cause of cancer-related deaths. Approximately 85% of CRCs arise from adenomas via the adenoma-carcinoma pathway.1 CRC screening has been implemented in several countries because early detection of CRC and removal of adenomas are considered to reduce the incidence and mortality of CRC. Therefore, removal of all adenomas is generally recommended for colonoscopy. However, most non-neoplastic polyps (e.g., hyperplastic polyps) of the colon do not develop into cancer. These polyps do not require removal; however, some or most are removed in clinical practice because the differentiation of neoplastic changes using endoscopy (i.e., optical diagnosis) is considered challenging. This leads to considerable costs and consumption of healthcare resources.2,3
Recently, computer-assisted polyp characterization (computer-aided diagnosis, CADx) has become a possible tool for implementing optical diagnosis by increasing confidence in diagnosis and decreasing the rate of removal of non-neoplastic lesions (Fig. 1). However, CADx can be performed using different tools produced by a variety of manufacturers, and not all CADx tools have sufficient accuracy to be implemented in healthcare according to the current standards. In this review, we examine the results of prospective trials and evaluate the possibility of using CADx tools in clinical practice.
OPTICAL DIAGNOSIS: GOALS AND CHALLENGESIn the previous 30 years, efforts have been made to improve the quality and endoscopistsŌĆÖ confidence in optical diagnosis. The application of indigo carmine and methylene blue to help evaluate surface structure and pit patterns could be an attractive option for this purpose,4 while recent virtual chromoendoscopy technologies, such as narrow-band imaging (NBI), allow more user-friendly optical diagnosis.5 However, optical diagnosis has not been widely disseminated, except in a limited number of countries and centers. Several factors may have affected this situation, including the fear of causing harm. Possible harm includes incorrect recommendations of surveillance intervals, risk of leaving malignant lesions due to incorrect evaluations, and possible liability issues caused by optical diagnosis-driven decision-making.
To overcome these barriers, academic societies have proposed several ŌĆ£standardsŌĆØ that endoscopists must follow when introducing optical diagnosis in colonoscopy. These standards include the preservation and incorporation of valuable endoscopic innovations (PIVI 1 and 2) criteria proposed by the American Society for Gastrointestinal Endoscopy (ASGE) and the Simple Optical Diagnosis Accuracy (SODA 1 and 2) criteria proposed by the European Society of Gastrointestinal Endoscopy (ESGE) (Table 1).6,7 Examples of these polyp handling strategies for optical diagnosis include the following: (1) ŌĆ£Leave-in-situ strategyŌĆØ7: Diminutive polyps (Ōēż5 mm) in the rectosigmoid predicted as non-neoplastic with high confidence are left in situ, while the other polyps are removed and assessed histologically. A negative predictive value (NPV) of >90% for identifying neoplastic changes is required to implement this strategy. (2) ŌĆ£Resect-and-discardŌĆØ7: Diminutive polyps predicted as neoplastic with high confidence are resected without being sent for histopathology. Optical diagnosis information is used to predict the surveillance intervals. Endoscopists must provide >90% agreement in surveillance interval recommendations between histology-based determination and optical diagnosis-based prediction. (3) ŌĆ£DISCARDŌĆØ-lite8: This strategy is a modification of the resect-and-discard strategy considering that most of the diminutive polyps on the right side should be either adenomas or sessile serrated lesions. All diminutive polyps in the proximal colon (between the cecum and descending colon) are assumed to be neoplastic and thus removed and discarded without pathological assessment. In addition, diminutive polyps in the rectosigmoid region, predicted to be non-neoplastic with high confidence, are left in situ.
Although these optical diagnostic strategies have specific threshold levels, many endoscopists still lack confidence in their ability to implement optical diagnosis.9 To overcome this barrier, the use of artificial intelligence in the form of CADx has attracted considerable attention, owing to its potential to provide endoscopists with confidence in optical diagnosis.10
ARTIFICIAL INTELLIGENCE FOR OPTICAL DIAGNOSISCADx tools in endoscopy utilize machine learning methods to classify images into specific categories, such as neoplastic versus non-neoplastic, which facilitates the endoscopistŌĆÖs optical diagnostic process.11 While several preclinical studies have been published in this field, clinical studies in which CADx tools have been used and evaluated in real-time are limited. Table 2 shows eight representative prospective studies that evaluated the performance of CADx in clinical colonoscopy.10,12-19 To date, there have been no published randomized trials in this academic field, and only two well-designed comparative prospective studies have been conducted. In this review, we elaborate on these two comparative studies because they provide dedicated knowledge on how the use of CADx affects standard optical diagnosis procedures.10,12
ITALIAN SINGLE CENTER PROSPECTIVE TRIALBlue light imaging (BLI; Fujifilm Corp.) is an image-enhanced technology similar to NBI that emphasizes the vascular and structural patterns of polyp surfaces. Fujifilm Corp. recently introduced a CADx tool designed to interpret BLI images in the market in Europe, Japan, and several other areas of the world (CAD EYE; Fujifilm Corp.). CAD EYE provides a binary prediction of polyp histology (neoplastic or non-neoplastic).
Rondonotti et al.12 conducted an observational clinical trial to assess whether BLI with CADx software was useful in colonoscopy by comparing the optical diagnostic performance of the following three groups: (1) endoscopists alone, (2) CADx alone, and (3) endoscopists using CADx. The primary endpoint was whether CADx-assisted optical diagnosis had Ōēź90% NPV for adenomatous histology, with histopathology as the reference point. This NPV threshold is one of the standards for optical diagnosis proposed by the ASGE (Table 1). Secondary endpoints were whether the endoscopists alone or CADx alone managed to reach this standard and the threshold level for the resect-and-discard strategy proposed by the ASGE (Table 1).
A total of 389 patients were included in the study. These patients had 596 diminutive polyps in the rectosigmoid that were subject to analysis, of which 259 were neoplastic and 337 were non-neoplastic. The NPV, sensitivity, and specificity were 90.9% (95% confidence interval [CI], 86.8ŌĆŖŌĆō93.7), 88.6% (95% CI, 83.6ŌĆō92.2), and 88.8% (95% CI, 84.5ŌĆō91.9), respectively, in group 1. However, the diagnostic performances of group 2 were 86.7ŌĆŖ% (95% CI, 82.3ŌĆō90.1), 81.9ŌĆŖ% (95% CI, 76.2ŌĆō86.5), and 88.7% (95% CI, 84.4 ŌĆō91.9), respectively. Group 3 achieved 91.0% (95% CI, 87.1ŌĆō93.9), 88.6% (95% CI, 83.7ŌĆō91.4), and 88.1% (83.9ŌĆō91.4), respectively. Agreement with post-polypectomy surveillance intervals according to the US recommendations was 92.6% (95% CI, 90.0ŌĆō95.2), 92.1% (95% CI, 89.4ŌĆō94.8), and 92.6% (95% CI, 90.0ŌĆō95.2) in groups 1, 2, and 3, respectively20; in contrast, that for the European recommendations was 97.1% (95% CI, 95.4ŌĆō98.8), 96.8% (95% CI, 95.0ŌĆō98.6), and 97.4% (95% CI, 95.7ŌĆō98.9), respectively.21 ŌĆŖ
The study showed that CADx-assisted optical diagnosis outperformed the threshold levels proposed by the ASGE and ESGE. However, this study did not provide convincing data on the added value of using CADx compared with optical diagnosis by endoscopists alone.
AN INTERNATIONAL MULTICENTER PROSPECTIVE TRIALThe endocytoscope, a high-resolution magnification colonoscope (CF-H290ECI; Olympus Corp.), can provide 520-fold magnification of a lesion that enables the evaluation of microvascular morphology. CADx software with this tool is commercially available in Japan and several Asian countries (EndoBRAIN; Cybernet Systems Corp.). EndoBRAIN predicts binary histology, namely neoplastic vs. non-neoplastic.
Barua et al.10 conducted a clinical study to assess whether the endocytoscope with CADx software could positively affect optical diagnosis compared with optical diagnosis by endoscopists alone. The study was performed in two sequential steps: (1) endoscopists alone and (2) endoscopists performing CADx. The primary endpoint was to compare the sensitivity of identifying diminutive adenomas in the rectosigmoid region between optical diagnoses with and without CADx. The results from optical diagnosis were then compared to the gold standard, namely histopathological diagnosis, with neoplastic lesions being evaluated as either adenomas or sessile serrated adenomas in primary analyses and non-neoplastic lesions as hyperplastic or other benign tissues.
In total, 518 patients were included in the final analysis. A total of 892 diminutive polyps in the rectosigmoid region were analyzed, of which 359 were neoplastic and 533 were non-neoplastic. The NPV, sensitivity, and specificity of optical diagnosis by endoscopists alone were 91.5% (95% CI, 88.5ŌĆō93.8), 88.4% (95% CI, 84.3ŌĆō91.5), and 83.1% (95% CI, 79.2ŌĆō86.4), respectively. However, the endoscopists using CADx achieved 92.8% (95% CI, 90.1ŌĆō94.9), 90.4% (95% CI, 86.8ŌĆō93.1), and 85.9% (95% CI, 82.3ŌĆō88.8), respectively. This study showed no significant increase in sensitivity when endoscopists used CADx for optical diagnosis. In contrast, the use of CADx significantly increased the confidence level in optical diagnosis from 74.2% (95% CI, 70.9ŌĆō77.3) to 92.6% (95% CI, 90.6ŌĆō94.3), which may contribute to the reduction of healthcare costs, given that optical diagnosis is usually performed only with high-confidence prediction.
DISCUSSIONTwo comparative studies showed that endoscopists alone and endoscopists using CADx outperformed most threshold standards for optical diagnosis. These studies have recently brought additional knowledge to this academic field.
First, these two studies highlighted the importance of confidence levels in optical diagnosis. High-confidence prediction is mandatory for optical diagnosis according to the ASGE/ESGE guidelines. However, previous studies evaluating CADx tools have not focused on this value. Barua et al.10 showed that CADx improved the confidence in performing optical diagnosis, which may affect the number of unnecessary polypectomies and histopathological assessments, as well as the cost of colonoscopy. However, an objective definition of high-confidence prediction is extremely difficult. This may depend on the personalities or cultures of the endoscopists. We expect that future studies will clarify the value of high-confidence diagnoses considering cultural backgrounds.
Second, the potential role of CADx tools in training inexperienced endoscopists should be further discussed.22 Rondonotti et al.12 found that CADx software provided non-experts with a rapid learning curve. However, the contribution of CADx to optical diagnosis training remains uncertain, given that it was not evaluated as a primary outcome measure in this study. Given that CADx helps in the interpretation of the polyp image but not the acquisition of a high-quality polyp image (which is a prerequisite for accurate optical diagnosis), further studies are needed. In addition, endoscopistsŌĆÖ reliance on CADx may hamper the development of optical diagnostic skills of endoscopists.
Furthermore, meeting the threshold levels proposed by ASGE/ESGE is only part of the CADx contribution: there is a need to comprehensively evaluate this innovative technology with a focus on the balance between its potential benefits and harms. This includes an analysis of the cost-effectiveness of CADx in several healthcare settings. Understanding the balance of using CADx (which is usually subtle in medicine) will guide the implementation of this innovative technology.
CONCLUSIONSCADx for colonoscopy is expected to optimize optical diagnosis for the assessment of small polyps, eventually leading to reduction of unnecessary polypectomies and relevant cost. However, the currently available, most reliable, prospective studies casted a question against its contribution to clinical practice. Further improvement of the artificial intelligence models together with convincing clinical testing is of great need.
NOTESFig.┬Ā1.Example of computer-assisted polyp characterization (CADx) with EndoBRAIN (Cybernet Systems Corp.). NBI, narrow-band imaging. 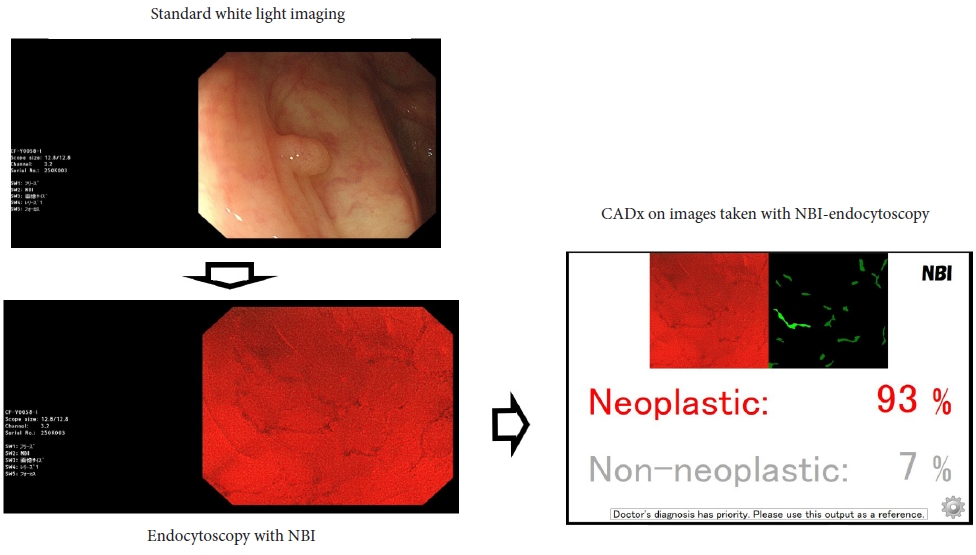
Table┬Ā1.
Table┬Ā2.Prospective studies using CADx in diminutive lesions
REFERENCES1. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16:713ŌĆō732.
2. Vleugels JL, Greuter MJ, Hazewinkel Y, et al. Implementation of an optical diagnosis strategy saves costs and does not impair clinical outcomes of a fecal immunochemical test-based colorectal cancer screening program. Endosc Int Open 2017;5:E1197ŌĆōE1207.
3. Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol 2010;8:865ŌĆō869.
4. Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol 1994;47:880ŌĆō885.
5. Rees CJ, Rajasekhar PT, Wilson A, et al. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut 2017;66:887ŌĆō895.
6. Houwen BB, Hassan C, Coup├® VM, et al. Definition of competence standards for optical diagnosis of diminutive colorectal polyps: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2022;54:88ŌĆō99.
7. Rex DK, Kahi C, O'Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011;73:419ŌĆō422.
8. Atkinson NS, East JE. Optical biopsy and sessile serrated polyps: is DISCARD dead? Long live DISCARD-lite! Gastrointest Endosc 2015;82:118ŌĆō121.
9. Willems P, Djinbachian R, Ditisheim S, et al. Uptake and barriers for implementation of the resect and discard strategy: an international survey. Endosc Int Open 2020;8:E684ŌĆōE692.
10. Barua I, Wieszczy P, Kudo SE, et al. Real-time artificial intelligence-based optical diagnosis of neoplastic polyps during colonoscopy. NEJM Evid 2022;1:EVIDoa2200003.
11. Chan HP, Hadjiiski LM, Samala RK. Computer-aided diagnosis in the era of deep learning. Med Phys 2020;47:e218ŌĆōe227.
12. Rondonotti E, Hassan C, Tamanini G, et al. Artificial intelligence-assisted optical diagnosis for the resect-and-discard strategy in clinical practice: the Artificial intelligence BLI Characterization (ABC) study. Endoscopy 2023;55:14ŌĆō22.
13. Aihara H, Saito S, Inomata H, et al. Computer-aided diagnosis of neoplastic colorectal lesions using 'real-time' numerical color analysis during autofluorescence endoscopy. Eur J Gastroenterol Hepatol 2013;25:488ŌĆō494.
14. Kuiper T, Alderlieste YA, Tytgat KM, et al. Automatic optical diagnosis of small colorectal lesions by laser-induced autofluorescence. Endoscopy 2015;47:56ŌĆō62.
15. Rath T, Tontini GE, Vieth M, et al. In vivo real-time assessment of colorectal polyp histology using an optical biopsy forceps system based on laser-induced fluorescence spectroscopy. Endoscopy 2016;48:557ŌĆō562.
16. Kominami Y, Yoshida S, Tanaka S, et al. Computer-aided diagnosis of colorectal polyp histology by using a real-time image recognition system and narrow-band imaging magnifying colonoscopy. Gastrointest Endosc 2016;83:643ŌĆō649.
17. Mori Y, Kudo SE, Misawa M, et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: a prospective study. Ann Intern Med 2018;169:357ŌĆō366.
18. Horiuchi H, Tamai N, Kamba S, et al. Real-time computer-aided diagnosis of diminutive rectosigmoid polyps using an auto-fluorescence imaging system and novel color intensity analysis software. Scand J Gastroenterol 2019;54:800ŌĆō805.
19. Minegishi Y, Kudo SE, Miyata Y, et al. Comprehensive diagnostic performance of real-time characterization of colorectal lesions using an artificial intelligence-assisted system: a prospective study. Gastroenterology 2022;163:323ŌĆō325.
20. Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158:1131ŌĆō1153.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
