AbstractBackground/AimsStricture formation is a common complication after endoscopic mucosal resection. Predictors of stricture formation have not been well studied.
MethodsWe conducted a retrospective, observational, descriptive study by using a prospective endoscopic mucosal resection database in a tertiary referral center. For each patient, we extracted the age, sex, lesion size, use of ablative therapy, and detection of esophageal strictures. The primary outcome was the presence of esophageal stricture at follow-up. Multivariate logistic regression was used to analyze the association between the primary outcome and predictors.
ResultsOf 136 patients, 27% (n=37) had esophageal strictures. Thirty-two percent (n=44) needed endoscopic dilation to relieve dysphagia (median, 2; range, 1 to 8). Multivariate logistic regression analysis showed that the size of the lesion excised is associated with increased odds of having a stricture (odds ratio, 1.6; 95% confidence interval, 1.1 to 2.3; p=0.01), when controlling for age, sex, and ablative modalities. Similarly, the number of lesions removed in the index procedure was associated with increased odds of developing a stricture (odds ratio, 2.3; 95% confidence interval, 1.3 to 4.2; p=0.007).
INTRODUCTIONThe incidence of esophageal adenocarcinoma (EAC) and high-grade dysplasia (HGD) found in Barrett esophagus (BE) has been increasing.1,2 Esophagectomy, once a traditional therapy for HGD and intramucosal EAC, has been associated with serious morbidity and mortality.3,4,5 Therefore, endoscopic therapy has been increasingly used to treat HGD and early mucosal adenocarcinoma in many institutions. Such endoscopic therapy usually consists of endoscopic mucosal resection (EMR) of the suspicious lesions, followed by ablation therapy to treat any residual dysplasia. EMR is also used to histologically stage the tumor. Many ablative therapies are now in use, including radiofrequency ablation (RFA), cryotherapy, photodynamic therapy (PDT), and argon plasma coagulation (APC).6,7,8,9,10 Esophageal stricture formation is one of the most common complications and can occur in up to 70% of patients.11 Lesions with a >50% esophageal circumference and a patient history of tobacco use were associated with an increased likelihood of stricture formation.12 Several other factors may influence stricture formation, such as the size and number of resected lesions and the type of ablative modality employed. Our study aimed to investigate the potential effects of lesion size and ablation technique on the risk of post-EMR stricture formation.
MATERIALS AND METHODSData collectionWe conducted a retrospective, observational, descriptive study by using a prospective EMR database. Our database contained information on 175 patients who had undergone esophageal EMRs at a tertiary referral center from May 2003 to June 2010. Patients were usually referred because of a diagnosis of dysplasia or nodules in addition to BE. Patients were included if they had at least one follow-up esophagogastroduodenoscopy (EGD) after the EMR or if a procedural complication, including dysphagia, was reported by the patient or their primary physician. In addition to our database, we also used the electronic medical record. Extracted data included age, sex, size of the excision, aspirin/nonsteroidal anti-inflammatory drug use, previous diagnosis of BE, maximum BE segment length, histology of disease, use of ablative therapies pre- and post-EMR (including PDT, RFA, APC, and cryotherapy), EMR technique, number of EMRs (if applicable), history of esophagectomy, follow-up times, presence of esophageal strictures, severity of the strictures, need for dilation, and number of dilations needed. We used the largest reported dimension of the lesion on EGD as representing the lesion size. The primary outcome was the presence of esophageal strictures on follow-up EGD. Most of our patients had long-term follow-up at our institution. Otherwise, the patient's status was obtained from the referring physician's office.
Standard protocolPatients were first evaluated by an experienced gastroenterologist, by using a standard protocol that included a review of medical history and a computed tomography scan of the chest, abdomen, and pelvis. Patients were then evaluated by using EGD with advanced imaging modalities such as high-definition white light, narrow-band imaging, and probe-based confocal endomicroscopy to detect suspicious lesions such as raised nodules, discrete lesions, or flat lesions associated with abnormal mucosal or vascular features of neoplasia. At another appointment, patients underwent endosonography with staging of possible cancers and, if indicated, fine needle aspiration of lymph nodes suspicious for the presence of neoplasia. If there was no evidence of locally advanced disease, EMR was used to remove suspicious areas. Patients were considered for surgery if they had a confirmed diagnosis of EAC with positive deep resection margins on EMR, or if they had features suggestive of a risk for locally advanced disease. For patients who had undergone piecemeal resection and who had carcinoma at the edge of one of the pieces, surgical triage was individualized based on the endoscopic assessment of whether the endoscopist felt that a radical resection (R0) had been achieved since the true lateral margins could not be assessed. For patients who did not undergo surgery, surveillance was performed at 2- and 3-month intervals with ablative therapy and biopsy or EMR of any remaining metaplasia. After complete eradication of Barrett metaplasia, patients received endoscopy with surveillance biopsy of the neosquamous epithelium at yearly intervals.
Endoscopic mucosal resectionMethods of EMR have been described in detail.13 Most EMR procedures were performed with the multiband mucosectomy method (DT-6-5F; Cook Medical, Bloomington, IN, USA) to remove the most suspicious lesions. For lesions suspected or proven to be an adenocarcinoma, a "rosette pattern" of four to six additional resections was then performed to completely remove the tissue around the index resection margin (Fig. 1). Up to 75% of the lumen circumference can be resected. APC was used to ablate the mucosal bridges between these resections. Index lesions >3 cm in diameter were removed using the cap technique with Olympus accessories (K-008; Olympus America Inc., Center Valley, PA, USA). In a few patients, EMR was done by injection and a standard endoscopic snare, without the use of cap or band techniques.
Endoscopic ablation therapiesBefore ablation therapy, which included RFA, PDT, APC, and cryotherapy,10,13,14,15,16,17,18 patients were prescribed high doses of a proton pump inhibitor medication, such as 40 mg omeprazole by mouth twice daily, once before the morning and evening meals, to aggressively control acid reflux. Patients returned for treatment every 3 to 6 months until all esophageal glandular mucosa had been successfully ablated and replaced with a neosquamous epithelium. Surveillance endoscopy was then performed every 6 to 12 months thereafter to detect and treat any recurrent or residual Barrett mucosa.
Stricture severity and dilationBased on previous studies,19 a stricture was defined as a narrowing in the lumen of the esophagus, identified on follow-up endoscopy, regardless of the presence of dysphagia. The severity of esophageal strictures was classified as mild if it allowed easy passage of a standard endoscope (outer diameter 9.8 mm, Olympus GIF H-180; Olympus America Inc.). Moderate strictures were defined as those that allowed passage of the standard endoscope with some resistance. Severe strictures were those that did not allow the passage of the standard diagnostic endoscope. Most dilations were done with Savary-Gilliard dilators with a guide wire (Cook Medical) (Fig. 1). The size of the dilator used varied by patient and degree of stricture, following the "rule of three."
Statistical analysisWe used SAS version 9.2 (SAS Institute, Cary, NC, USA) for statistical analysis. For continuous variables, we used the Wilk-Shapiro test to assess normality. For normal variables, we reported means and standard deviations. For nonnormal data, we reported medians and interquartile ranges or ranges. For discrete data, we reported proportions. We used Student t-test to assess for differences between two means for normal data, whereas we used the Wilcoxon ranks sum test for continuous nonnormal data. We used Fisher exact or chi-square tests to assess for differences between proportions in different categories. We used univariate logistic regression analysis to assess the association between the primary outcome (presence of esophageal strictures) and potential predictors. Variables that reached significance in univariable analysis with p<0.2 were included in multivariate logistic regression analysis. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs) and p-values (<0.05 was used for statistical significance). This study was reviewed and approved by the Mayo Clinic Institutional Review Board.
RESULTSWe identified 136 patients who underwent EMR of esophageal lesions. Indications for the procedures included esophageal nodules or a history of HGD or EAC. All patients had at least one follow-up EGD. Overall, 27% (n=37) were found to have esophageal strictures. We reviewed the patient and procedure characteristics for the study population by dividing the cohort into two groups. The nonstricturing group consisted of 99 patients who did not develop strictures on follow-up EGD. The stricturing group consisted of 37 patients who developed esophageal strictures. The mean age for the nonstricturing group was similar to the stricturing group (70┬▒10 years vs. 72┬▒10 years, p=0.43). Most (>83%) of the patients in both groups were men. The median lesion size, number of EMR sessions, and follow-up times were similar between the two groups (Table 1).
The EMR methods were similar in both groups. The most common technique was the Duette system (multiband mucosectomy), which was used in >60% of the cases. The second most common technique was the cap-assisted technique, which was used in 24% of patients in the nonstricturing group and in 19% of patients in the stricturing group (p=0.51). The rosette technique, described earlier, was employed in a minority of patients: 8% in the nonstricturing group and 14% in the stricturing group. Nineteen percent of patients in the stricturing group had a history of post-EMR surgery compared with 10% of patients who did not develop strictures. Sixty-five percent of patients who developed strictures had a history of being treated with RFA compared with 56% of patients with no esophageal strictures. Twenty percent of patients who developed strictures had a history of treatment with PDT compared with 18% of patients who did not develop strictures. Patient characteristics are summarized in Table 1.
Post-EMR tissue diagnoses were most commonly adenocarcinoma (31% of nonstricturing vs. 41% in stricturing group) and HGD (29% in nonstricturing vs. 38% in the stricturing group). Other diagnoses were less common. Those are summarized in Table 2. If more than one lesion was resected with EMR in the index procedure, we reported the specimen with the highest grade of histology based on the modified Vienna classification.20
Among the 37 patients who developed esophageal strictures, all responded well to endoscopic dilation with resolution of their dysphagia. For patients who required dilation, the median number of dilations was two (range, 1 to 8). Mild strictures, allowing the passage of a regular-sized EGD scope, occurred in 13% (n=18) of all patients. Moderate or severe strictures occurred in 14% (n=19) of all patients. As shown in Table 1, univariate analysis of various EMR techniques showed no significant association with developing strictures. This was also true for the various ablative therapies (Table 1).
On univariable analysis, age, sex, PDT, and RFA did not have a significant association with stricture formation (Table 3). Based on our preset criteria, the number of lesions, the size of the lesion, and a history of esophageal surgery were included in a multivariate logistic regression analysis. Lesion size was associated with increased odds of having a stricture (OR, 1.5; 95% CI, 1.1 to 2.0; p=0.027). This means that for each 1 cm increase in lesion size, there was a 50% increase in the odds of developing esophageal stricture. Similarly, the number of lesions removed in the initial procedure was associated with increased odds of developing a stricture (OR, 2.1; 95% CI, 1.2 to 3.6; p=0.027). Therefore, for each additional lesion removed on EMR, the odds of developing esophageal stricture doubled.
DISCUSSIONRecent data suggest that the incidence of EAC has been increasing.1,2 Similarly, endoscopic options for managing HGD or early adenocarcinoma of the esophagus have become more common. Many patients, especially the elderly, are often not suitable candidates for surgery and may prefer endoscopic therapy. The use of EMR for the treatment and staging of esophageal disease is now well described. Major centers in the United States and Europe offer endoscopic therapy as a first-line therapy for intramucosal EAC. Ablative therapies with RFA, PDT, and cryotherapy have also been well studied and published.7,8,12,13,14,16,18
As reported here, esophageal strictures are among the most common complications after esophageal EMR. Several studies have estimated the rate of post-EMR strictures to be 4% to 70%.11,21,22,23,24,25,26 To our knowledge, only a few studies have looked at possible predictors of strictures.12,25 Prasad et al.25 reviewed 131 patients with HGD who underwent post-EMR PDT. They reported a stricture rate of 27%, which is similar to what we report in this study. Their study, however, focused on patients who have had PDT. They found that EMR before PDT was a predictor of stricture formation. In our study, PDT was done in a total of 27 patients. As seen in univariable analysis, neither PDT nor RFA caused an increased risk of stricture formation in our patient cohort. This may be due to the smaller number of patients who had PDT in our population as compared with the study of Prasad et al.25
Our current study showed that the size of the EMR is correlated with an increased risk of stricture development, independent of the EMR technique and the number of lesions removed. These results echo the findings by Lewis et al.,12 who reported that the number of lesions resected was associated with increased risk for strictures. The results are also in agreement with Katada et al.,27 who found a higher rate of strictures if the EMR involved >75% of the esophageal circumference. In those studies, this association was only found on a univariate analysis, which is prone to confounding. To our knowledge, our study is the first to confirm the above findings based on a multivariate analysis controlling for possible confounders.
A more recent study by Chung et al.26 focused on strictures that occur after complete Barrett resection. They showed that the number of resections on the initial EMR was associated with increased odds of developing stricture (OR, 1.6). This is very similar to our OR of 2.1. However, our cohort included mostly patients who underwent EMR followed by ablative therapy, whereas Chung et al. studied patients who had EMR only with complete Barrett eradication (CBE). In practice, many patients undergo EMR and ablative therapy. Therefore, we believe that our analysis may be more reflective of current treatments and that it offers insight to the predictors of stricture formation in those patients. Interestingly, the percentage of patients who needed esophageal dilation in the Chung study was 33%. This is very similar to the percentage of patients who needed dilation in our study (32%). Based on the Katada study, one may have expected higher rates of strictures in patients who undergo CBE. However, our data suggest that EMR followed by ablative therapy may have a similar rate of stricture formation as that of CBE. Therefore, CBE may be a safe alternative to EMR and ablative therapy.
The rosette pattern of four to six additional resections, after the initial band-assisted EMR, was performed in a minority of patients in this cohort. This usually resulted in resection of up to 75% of the lumen circumference. Based on the above results, we expected to find an increased risk of stricture formation with this technique when compared to others. Therefore, we performed a subanalysis to estimate the risk of strictures in patients who have undergone this technique while controlling for age, sex, size of lesion, and ablation technique. There was a trend of more strictures in the rosette group; however, it was not statistically significant (OR, 1.6; 95% CI, 0.5 to 5.9; p=0.4). This is likely due to the small number of patients who underwent this procedure (n=14).
Many patients had more than one suspicious area that was resected in the initial procedure. In this study, we showed that resection of each additional lesion on the index procedure doubles the odds of esophageal stricture formation. Based on these results, if multiple EMRs are needed, the lesions appear to be low risk (no dysplasia or low-grade dysplasia on biopsies), and the patient is at low risk of adenocarcinoma (younger patients, nonsmokers, no family history of esophageal cancer), the clinician could consider doing EMRs on separate sessions. This may reduce the risk of stricture formation. However, such association needs to be further evaluated in a prospective manner. In addition, for patients at high risk of esophageal cancer (biopsies showing adenocarcinoma or HGD, older patients, history of smoking, family history of esophageal cancer), all suspicious lesions should be resected at the index procedure knowing that there is an increased risk of esophageal stricture formation. The rate of stricture formation in our cohort was similar to other studies with EMR followed by ablation.25,28 In addition, all strictures were successfully treated with endoscopic dilation. In all patients, this resulted in the resolution of symptoms but did not cause any complications (bleeding or perforation).
Of note, some patients had post-EMR esophagectomy. These were patients who had adenocarcinoma, HGD, and who elected to have surgery. Data reporting and analysis on those patients were done before the esophagectomy, as some patients had esophagectomy long after EMR. Some had esophageal strictures after EMR but before esophagectomy; they were included and analyzed in this study. Once the esophagus is removed, esophageal strictures cannot be analyzed.
Our study has several limitations, including the retrospective design, small sample size, and the fact that it was done in a single tertiary care center. We did not have reliable information on the percentage of esophageal circumference that was resected on all patients. Therefore, we did not analyze for this outcome specifically. However, intuitively, the size of the lesion and the number of resections are correlated with the circumferential area of resection (the bigger the size and the more resections, the higher the percentage of circumference resected). Therefore, our larger study confirms the results of the studies by Lewis et al.12 and Chung et al.26 Additionally, injection of steroids to treat or prevent esophageal strictures has become a hot topic recently.29,30 None of our patients received steroid injections; therefore, this issue was not discussed in our study. As more evidence accumulates, steroids may be studied and used. Another limitation is the lack of information about the presence and size of hiatal hernias in this cohort. Having recognized the above limitations, this study is, to our knowledge, the largest study to address the predictors of esophageal stricture formation, and the results of the study are an important contribution to the existing body of literature about this important complication.
In conclusion, we have found that post-EMR and postablative therapy stricture formation is the most common complication. Removing larger lesions and removing several lesions in the index procedure seem to be associated with a higher risk of developing esophageal strictures. The effect of post-EMR ablative modality seems similar among the different techniques, with PDT and RFA showing a trend toward more stricture formation when used post-EMR. Larger prospective studies are needed to assess the risks of post-EMR and post-RFA stricture formation compared with CBE.
References1. Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 2002;11:235ŌĆō256. 12424848.
2. Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer 2002;99:860ŌĆō868. 12115489.
3. Clark GW, DeMeester TR. Surgical management of Barrett's esophagus. Ann Chir Gynaecol 1995;84:139ŌĆō144. 7574371.
4. H├Člscher AH, Bollschweiler E, Schneider PM, Siewert JR. Early adenocarcinoma in Barrett's oesophagus. Br J Surg 1997;84:1470ŌĆō1473. 9361616.
5. Pera M, Trastek VF, Carpenter HA, Allen MS, Deschamps C, Pairolero PC. Barrett's esophagus with high-grade dysplasia: an indication for esophagectomy? Ann Thorac Surg 1992;54:199ŌĆō204. 1637206.
6. Pacifico RJ, Wang KK, Wongkeesong LM, Buttar NS, Lutzke LS. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett's esophagus. Clin Gastroenterol Hepatol 2003;1:252ŌĆō257. 15017665.
7. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc 2007;65:3ŌĆō10. 17185072.
8. Sampliner RE. Endoscopic therapy for Barrett's esophagus. Clin Gastroenterol Hepatol 2009;7:716ŌĆō720. 19306943.
9. Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc 2008;68:35ŌĆō40. 18355819.
10. Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 2011;141:460ŌĆō468. 21679712.
11. Soehendra N, Seewald S, Groth S, et al. Use of modified multiband ligator facilitates circumferential EMR in Barrett's esophagus (with video). Gastrointest Endosc 2006;63:847ŌĆō852. 16650552.
12. Lewis JJ, Rubenstein JH, Singal AG, Elmunzer BJ, Kwon RS, Piraka CR. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett's esophagus. Gastrointest Endosc 2011;74:753ŌĆō760. 21820109.
13. Wolfsen HC. Endoluminal therapy for esophageal disease: an introduction. Gastrointest Endosc Clin N Am 2010;20:1ŌĆō10. 19951790.
14. Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc 2010;71:686ŌĆō693. 20363410.
15. Gross SA, Wolfsen HC. The role of photodynamic therapy in the esophagus. Gastrointest Endosc Clin N Am 2010;20:35ŌĆō53. 19951793.
16. Panossian AM, Raimondo M, Wolfsen HC. State of the art in the endoscopic imaging and ablation of Barrett's esophagus. Dig Liver Dis 2011;43:365ŌĆō373. 21330224.
17. Wolfsen HC. Endoscopic ablation therapy: imaging and advanced technology in action. Gastroenterology 2009;137:1225ŌĆō1228. 19703456.
18. Wolfsen HC, Hemminger LL, Wallace MB, Devault KR. Clinical experience of patients undergoing photodynamic therapy for Barrett's dysplasia or cancer. Aliment Pharmacol Ther 2004;20:1125ŌĆō1131. 15569115.
19. Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277ŌĆō2288. 19474425.
20. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251ŌĆō255. 10896917.
21. Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200ŌĆō1206. 18460553.
22. Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut 2010;59:1169ŌĆō1177. 20525701.
23. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 2009;137:815ŌĆō823. 19524578.
24. Seewald S, Ang TL, Gotoda T, Soehendra N. Total endoscopic resection of Barrett esophagus. Endoscopy 2008;40:1016ŌĆō1020. 19065485.
25. Prasad GA, Wang KK, Buttar NS, Wongkeesong LM, Lutzke LS, Borkenhagen LS. Predictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett's esophagus. Gastrointest Endosc 2007;65:60ŌĆō66. 17185080.
26. Chung A, Bourke MJ, Hourigan LF, et al. Complete Barrett's excision by stepwise endoscopic resection in short-segment disease: long term outcomes and predictors of stricture. Endoscopy 2011;43:1025ŌĆō1032. 22068701.
27. Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003;57:165ŌĆō169. 12556777.
28. Peters FP, Kara MA, Rosmolen WD, et al. Stepwise radical endoscopic resection is effective for complete removal of Barrett's esophagus with early neoplasia: a prospective study. Am J Gastroenterol 2006;101:1449ŌĆō1457. 16863545.
Fig.┬Ā1Endoscopic mucosal resection with the "rosette" technique. (A) Esophageal nodule in the background of Barrett's esophagus as seen in high-definition white light. (B) Same nodule as seen in narrow-band imaging. (C) Mucosal defect after resection of the nodule. (D) Large mucosal defect after completion of the rosette resection. (E) Specimens embedded on paraffin wax. (F) Endoscopic view of an esophageal stricture post endoscopic mucosal resection (before dilation). 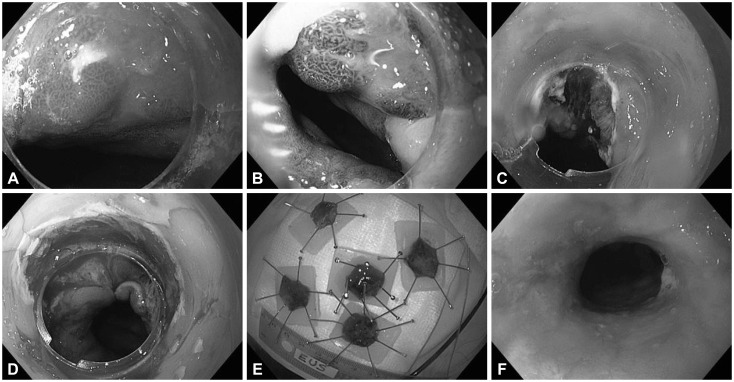
|
|
|||||||||||||||||||||||||||||||||||||||||||||||
