INTRODUCTION
Endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) is a safe, effective, and minimally invasive alternative to percutaneous gallbladder drainage (PTGBD) for acute cholecystitis. In recent years, EUS-GBD has become a simple and safe procedure owing to advances in techniques and the expansion of indications.1
Plastic stents (PS) or endoscopic nasobiliary drainage (ENBD) tubes have been formerly used for EUS-GBD. However, self-expandable metallic stents, especially lumen-apposing metal stents, have recently gained popularity.1-3 Technical failures during EUS-GBD performed by endoscopists with limited experience often cause insertion failure of a fistula dilating device, difficulties with the delivery system when deploying the stent, or misplacement of the stent in the gallbladder during the last step.4
To overcome these challenges, we have developed a novel single-pigtail plastic stent (SPPS) for EUS-GBD. SPPS was used in four patients with acute cholecystitis. We present the findings of this case study, which was undertaken to assess the technical feasibility and functional success rate of the new SPPS dedicated for use in EUS-GBD.
CASE REPORT
We retrospectively reviewed the cases of all four patients who underwent EUS-GBD using SPPS for acute cholecystitis from July 2019 to July 2021. The mean age of the patients was 82.3 years (range, 73ŌłÆ88 years), with a male-to-female ratio of 1:1. Acute cholecystitis was diagnosed based on the clinical findings of fever and epigastric and right upper quadrant pain. Abdominal computed tomography and/or magnetic resonance imaging were performed to confirm the diagnosis of cholecystitis and simulate EUS-GBD procedures.
Four patients with acute cholecystitis were selected for EUS-GBD using SPPS; three patients underwent EUS-GBD using SPPS as a fallback procedure from PTGBD for recurrent cholecystitis following blockage of the PTGBD tube (conversion cases, C cases); and one patient underwent direct EUS-GBD as this patient was a poor candidate for performing alternative procedures (direct case, D-case). In case D, we could not perform the PTGBD procedure due to ascites and fat deposits within the puncture route (Supplementary Video 1), and endoscopic transpapillary gallbladder drainage was difficult because of the unstable position of the scope due to the patientŌĆÖs obesity. The time between the PTGBD procedure and EUS-GBD ranged from 23 to 58 days in C cases. Patients were deemed at high risk for cholecystectomy if they satisfied one or more of the following criteria: age Ōēź80 years, American Society of Anesthesiology grade Ōēź3,5 age-adjusted Charlson comorbidity index >5,6 and prognostic nutritional index <47 (Table 1).7 All patients provided written informed consent to undergo EUS-GBD with SPPS. This study was approved by the Ethical Review Committee of the Omihachiman Community Medical Center (approval number: ERB-C-875) and was conducted in accordance with the Helsinki Declaration of the World Medical Association. Consent for publication was obtained from all patients.
To prepare an SPPS of sufficient length, a 7.5-Fr ENBD tube (Fig. 1A, Flexima; Boston Scientific) was cut to a length of 15 to 20 cm and side holes were added using a tube punch (Fig. 1B). We determined the appropriate SPPS length as follows: in cases where endoscopic manipulation could be performed, a 15-cm long SPPS was inserted; in cases where securing the ultrasound view and holding the endoscope position was difficult, a 20-cm long SPPS was inserted (Fig. 1C). Side holes were punched with an ethylene oxide-sterilized punch in a clean environment immediately before insertion to ensure aseptic SPPS preparation. To prevent early stent occlusion and SPPS contamination with food particles, holes were drilled 3 to 5 cm from the straight edge on the duodenal side of the tube. Sterile saline was injected into the SPPS to confirm clear passage and the usefulness of the fistulae. During SPPS insertion, the remaining ENBD tube was used as a pusher catheter along with a guidewire (Fig. 1C). When the SPPS is constructed from ENBD tubes, it has a narrower tip at the end that widens when compared to the double-pigtail plastic stent (DPPS), which improves insertion by reducing the possibility of the tube getting stuck in the intestinal lumen (Fig. 1D).
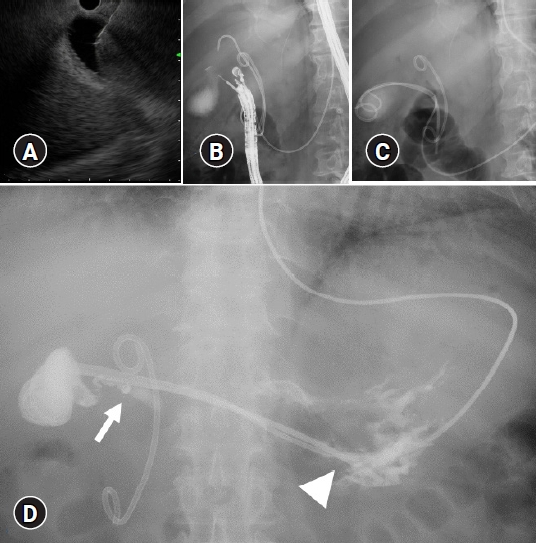
The patient received intravenous propofol and pentazocine hydrochloride for conscious sedation. The convex echoendoscope used for the procedures was GF-UCT260 (Olympus Medical System). The gallbladder was punctured from the duodenal bulb using a 19-G fine-needle (Ez shot 3 19G; Olympus Medical System) with EUS imaging (Fig. 2A). Gallbladder puncture was confirmed using contrast injection under fluoroscopy (Fig. 2B). A 0.025-inch guidewire (VisiGlide 2; Olympus Medical System) was then inserted through the needle and coiled inside the gallbladder lumen. The puncture route was dilated using a 4 to 8 mm biliary balloon dilator (Ren; Kaneka Medix Corp., Osaka, Japan), and a second 0.035-inch guidewire was inserted through a double-lumen cannula (UNEVEN; Piolax Medical Devices Inc.). During SPPS insertion, the remaining ENBD tube was used as a pusher catheter along with the guidewire. Following the completion of all EUS procedures, PTGBD drainage was reopened in the C cases. In case D, a 6-Fr pigtail ENBD tube (Boston Scientific) was temporarily inserted along with the SPPS (Fig. 2C, Supplementary Video 1). After the procedure, we performed gallbladder imaging via the PTGBD/endoscopic naso-EUS-GBD tube prior to tube removal (Fig. 2D).
EUS-GBD with SPPS was technically feasible and clinically successful. The procedure duration ranged from 20 to 42 min. All patients had their PTGBD/endoscopic naso-GBD tubes removed within 14 days of EUS-GBD. EUS-GBD fistula tract maturity was routinely assessed prior to auxiliary tube removal. Contrast imaging was performed in all four patients to confirm gastric/duodenal placement at the end of the SPPS tube. The SPPS spontaneously detached 413 and 57 days after the procedure in patients 1 and 4, respectively, with patient 1 developing acute cholangitis 426 days after the EUS-GBD. The patientŌĆÖs condition improved following the administration of antibiotics without requiring an interventional procedure. Patient four did not experience any complications related to cholecystitis. The remaining two patients had no cholecystitis recurrence during the follow-up periods of 841 and 900 days, respectively, after the procedure (Table 1).
DISCUSSION
We developed a novel SPPS from an ENBD tube and evaluated its feasibility and technical and functional success rates. Once the SPPS is firmly inserted into the gallbladder, it can be safely released by pushing the stent further, even if the SPPS flexes in the gastrointestinal tract when the endoscope is close to the wall of the punctured gastrointestinal tract, as shown in Supplementary Video 1.
Stent displacement or maldeployment is the most concerning complication of EUS-GBD.8 At the time of needle puncture, the direction of the guidewire entering the gallbladder is usually perpendicular to the gallbladder wall. However, excessive coiling of the indwelling guidewire within the gallbladder can result in greater loop formation and a change in the axis of the guidewire. This change can directly lead to procedural failure despite successful guidewire coiling within the gallbladder.4 Another crucial point pertaining to the technical aspects is that the stent may be deployed in the stomach or duodenum, or it may migrate outward into the peritoneum. Stent migration usually occurs when the endoscope is in an unstable position.8,9
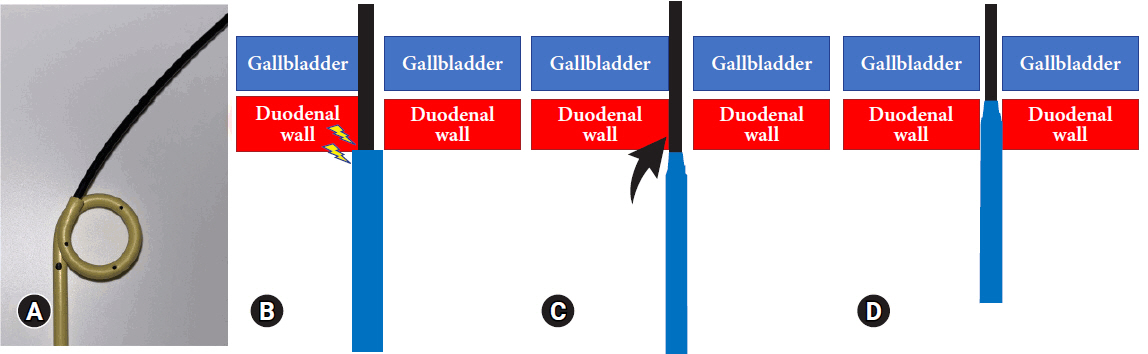
The tip shape of an SPPS is also an important factor in EUS-GBD. The gap between the inner sheath and DPPS (Fig. 3A) was caught at the edge of the intestinal wall, and DPPS was lost in the gallbladder (Fig. 3B). As depicted in Figure 1D, the SPPS was created using an ENBD tube, which has a narrower tip than DPPS tubes, to improve insertion by limiting instances in which the tube becomes lodged in the intestinal lumen during advancement (Fig. 3C). The tip shape promotes SPPS insertion because of the smaller difference in diameter between the guidewire and SPPS, thereby facilitating the advancement of the catheter through the duodenal wall (Fig. 3D). In addition, the SPPS is composed of Flexima material, which is created by improving the polymerization ratio of the polyurethane composition, which softens to 20% of its hardness at body temperature, thus avoiding undue stress on living tissue.10
Although SPPS is easily inserted into the gallbladder, it can be removed more easily than PS. We considered that the choice of SPPS during EUS-GBD in high-risk patients with inoperable cholecystitis1 should be based on the safety of stent placement and the benefit of internal fistula creation for a certain period rather than the risk of SPPS dislodgement. Although this is a small number of reported cases, the usefulness of EUS-GBD with SPPS can be seen. Additional studies with longer follow-up periods are necessary to establish the efficacy of these procedures and devices and to confirm our results.
In conclusion, we developed a novel SPPS from an ENBD tube and evaluated its feasibility, as well as its technical and functional success rates. This novel concept of adjusting the length, number, and location of the side holes of a PS according to a patientŌĆÖs anatomy for proper placement may be a useful option in EUS-GBD.