INTRODUCTION
Achalasia is an idiopathic neurodegenerative esophageal motility disorder characterized by impaired relaxation of the lower esophageal sphincter (LES) and the absence of normal peristalsis.1,2 Although the exact etiology of achalasia remains unknown, it may be associated with autoimmune, viral, and neurodegenerative factors.3 Inflammatory changes within the esophagus following these causative insults result in the loss of inhibitory neurons in the myenteric plexus of the distal esophagus and cause an imbalance between excitatory and inhibitory neurons, preventing the relaxation of the LES.4,5
The symptoms of achalasia may differ among patients; however, the major clinical manifestations include progressive dysphagia of both solids and liquids, chest pain, heartburn, regurgitation, vomiting, and weight loss.3 If untreated, achalasia can progress to a decompensated esophagus, eventually resulting in serious malnutrition.
The reported incidence and prevalence of achalasia vary by studies, with an annual incidence of 0.39 to 1.63 individuals per 100,000 and a prevalence of 7.8 to 10.82 per 100,000.6,7 Recent studies have noted an increase in the overall prevalence, probably due to the chronic nature of the disease, increased awareness, and improvements in diagnostic methods, especially in the application of high-resolution manometry (HRM).6,8,9
In this review, we elucidated the role of endoscopy in achalasia, including endoscopic diagnosis, treatment, and surveillance of the disease.
ENDOSCOPIC DIAGNOSIS OF ACHALASIA
Patients with suspected symptoms are diagnosed with achalasia using three major diagnostic modalities: HRM, esophagogastroduodenoscopy, and barium esophagography. Recently, a functional lumen imaging probe (EndoFLIP; Medtronic) was introduced as an innovative diagnostic tool that uses impedance planimetry to evaluate the esophageal geometry and measure the distensibility of the esophagogastric junction (EGJ).10
Although HRM is the gold standard for the diagnosis of achalasia, endoscopy plays an important role in early evaluation, primarily to exclude other diseases such as webs, rings, esophageal cancer, and proximal gastric cancer, which can mimic the symptoms of achalasia.1,11 The most common cause of secondary achalasia is malignancy, which accounts for more than half of all cases, followed by benign lesions and sequelae of surgical procedures.12 Eosinophilic esophagitis (EoE) is an important disease that must be ruled out. The symptoms of EoE are very similar to those of achalasia, and 33% to 56% of patients are reported to experience food impaction requiring endoscopic removal.13,14 Several studies have reported a possible association between achalasia and EoE in some patients, suggesting poorer outcomes in patients with achalasia in whom EoE is also present.15,16 Thus, endoscopic evaluation with multiple esophageal biopsies is recommended for patients with dysphagia to identify possible EoE.
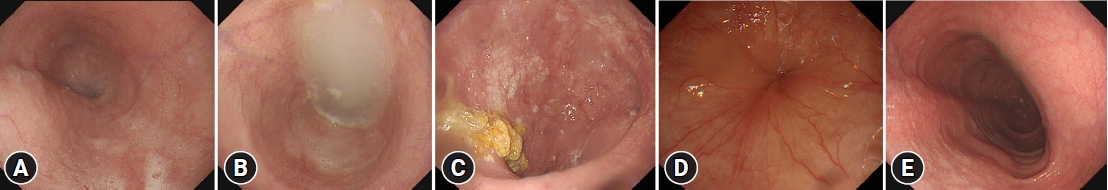
The classic endoscopic findings of achalasia include retained saliva with a puckered EGJ, widening of the esophageal lumen, and food residue in the esophagus.3,11 The descriptive rules for achalasia of the esophagus, established by the Japan Esophageal Society present the diagnostic features of achalasia on endoscopy as follows: (1) dilatation of the esophageal lumen; (2) abnormal retention of food and/or liquid remnants in the esophagus; (3) whitish change and thickening of the esophageal mucosal surface; (4) functional stenosis of the EGJ, in which the endoscope passes through the stenotic segment and the EGJ fails to dilate by insufflation; and (5) abnormal contraction waves of the esophagus (Fig. 1).17
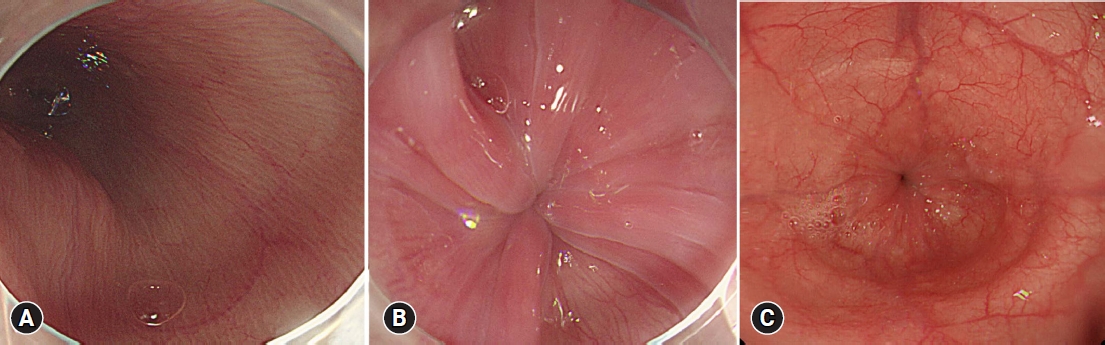
In early or type III achalasia, the esophageal mucosa may appear normal, which can lead to a greatly delayed diagnosis. Only approximately half of the patients show characteristic endoscopic features; therefore, caution should be taken not to exclude the diagnosis of achalasia in patients with normal findings on endoscopy.18 Several endoscopic features have recently been identified as highly indicative of achalasia (Fig. 2). Minami et al.19 reported minute superficial wrinkles, called the ŌĆ£pinstripe patternŌĆØ, on the esophageal surface of 60.7% of patients with achalasia, while dilatation of the esophageal lumen or remnant food material, known to be a characteristic feature of achalasia, was observed in 41.1% of the patients. Iwakiri et al.20 reported the endoscopic features of non-visibility of the esophageal palisade vessels (EPVs) and the appearance of rosette-like esophageal folds in the lower esophagus during deep inspiration as characteristic findings of primary achalasia. The authors reported the disappearance of EPVs in 28 of 34 patients and the formation of rosette-like esophageal folds in 33 of 34 patients. Gomi et al.21 suggested the champagne glass sign, defined when the distal end of the LES relaxation failure is proximal to the squamocolumnar junction (SCJ) and the SCJ is dilated in the retroflex view, as an endoscopic feature suggestive of achalasia. Further studies on the endoscopic features with larger sample sizes are warranted. Nonetheless, these findings may serve as helpful indicators of achalasia in some patients.
ENDOSCOPIC TREATMENT OF ACHALASIA
As no treatment can restore damaged esophageal function, options are limited to palliative treatments aimed at reducing LES resistance and facilitating food transit into the stomach.11 The classic treatment options in the past decades include pharmacological, endoscopic, and surgical approaches. The major endoscopic treatments currently available include botulinum toxin injections, endoscopic pneumatic balloon dilatation (PD), and peroral endoscopic myotomy (POEM). Surgical myotomy, introduced by Heller in 1913, was the most reliable treatment option for achalasia before the nonsurgical endoscopic treatment method POEM was introduced in 2010.22 Since then, POEM has become one of the most important minimally invasive treatment options for esophageal achalasia.
Intrasphincteric botulinum toxin injection was first introduced by Pasricha et al. in 1994 and was designed based on the idea that botulinum toxin inhibits the release of acetylcholine from nerve endings, which can eventually lead to a temporary relaxation of the LES.23,24 Pasricha et al.24,25 conducted a study wherein patients with achalasia were randomly enrolled into either botulinum toxin or placebo injection groups. The botulinum toxin injection group showed significantly better symptom improvement compared to the placebo group.
There remain no standard guidelines for botulinum injection, but it is largely conducted as first described by Pasricha et al.,24 in which 80 to 100 units of botulinum toxin are administered in each quadrant of the LES in divided doses.26 Although botulinum injection is a relatively safe and easy procedure with good short-term efficacy, its major drawback is poor durability. Previous studies reported significant symptom relief of 64% to 76.7% in patients with achalasia immediately after the procedure but a substantial decline in symptom improvement after 6 months.27-29 Therefore, botulinum toxin injection is generally not recommended as a primary treatment option but is mainly suggested in patients with high morbidity with difficulty undergoing more invasive procedures or as bridge therapy before undergoing other invasive treatments.
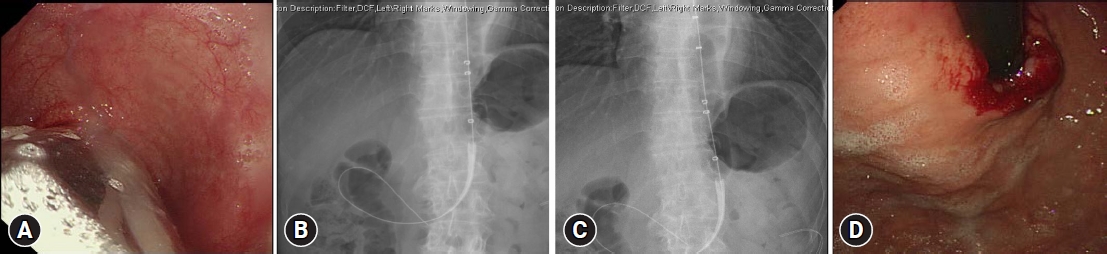
Endoscopic PD is a frequently used treatment method for achalasia and was the most effective nonsurgical treatment option before the introduction of POEM (Fig. 3). This procedure mechanically disrupts the LES muscle fibers by stretching an air-filled balloon.30 The balloon is positioned across the LES and inflated using fluoroscopy until waist obliteration is observed. A graded dilatation approach, starting with a 3-cm balloon and gradually increasing the balloon size by 5 mm when there is insufficient symptom relief, is recommended to minimize the risk of perforation during the procedure.3,31,32 PD provided relatively long-term symptom relief of over 86% after 2 years of follow-up.33 The potential complications of PD include bleeding, chest pain, and most importantly, perforation, which reportedly occurs in 2.9% to 4.3% of patients.34,35 Esophageal perforations may require surgical repair.
Several clinical factors are predictive of favorable outcomes in PD, including type II achalasia, old age, female sex, and low post-dilation LES pressures.36-38 The clinical success rate of PD differs according to the manometric subtype, and PD is not recommended for achalasia type III because of poor efficacy. Rohof et al.39 compared treatment outcomes between PD and Heller myotomy for each manometric subtype and observed comparably high success rates in type I and type II achalasia for both treatment groups, but impaired treatment response for PD in patients with type III achalasia.
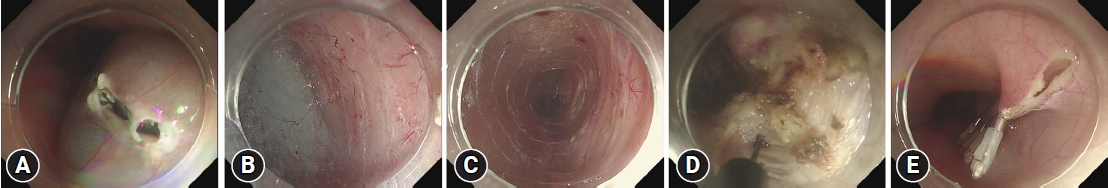
POEM was first introduced by Inoue et al.40 in 2010 and was inspired by the concept of the ŌĆ£natural orifice transluminal endoscopic surgeryŌĆØ approach to Heller myotomy. This approach aimed to combine the advantages of a minimally invasive endoscopic approach and the long-term efficacy of surgical myotomy. POEM is generally performed as a four-step procedure; namely, entry into the submucosal space, submucosal tunneling, endoscopic myotomy, and closure of the mucosal entry (Fig. 4).41
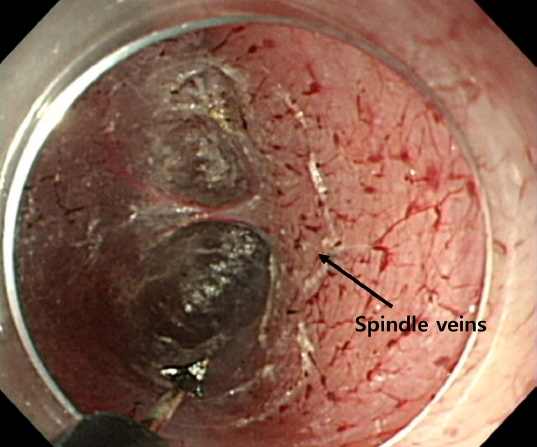
The first step is entering the submucosal space by creating an approximately 2 cm-long longitudinal mucosal incision 13 cm proximal to the EGJ. Once the incision is made, submucosal tunneling just above the muscle layer is performed using a technique similar to that used for submucosal endoscopic dissection. It is important to dissect deep enough to determine the direction of the circular muscle fibers because maintaining a direction perpendicular to the circular muscle is essential to avoid unintentional deviation of the submucosal tunneling and prevent damage to the mucosa above the tunnel. After creating a long submucosal tunnel 2 to 3 cm distal to the EGJ, the muscle bundle is dissected toward the stomach and 2 to 3 cm into the cardia. While accurately identifying the anatomy of the EGJ is important, defining its exact location is difficult. The landmarks of the EGJ include the length of the inserted endoscope, narrowing of the submucosal space at the level of the EGJ, and the presence of palisade vessels on the underside of the esophageal mucosal flap, spindle veins in the gastric cardia, large perforating vessels on the muscular side of the stomach, and blue discoloration of the cardia on retroflexion.41,42 Spindle veins are small, twisted veins with ampullar bulges seen in the submucosal layer just beneath the LES and are reported in 70% of patients with achalasia (Fig. 5).43 Recently, Grimes et al.44 introduced double-scope POEM, wherein a second endoscope is used to obtain a retroflexed view of the cardia, while the first scope is placed at the end of the submucosal tunnel to transilluminate and precisely identify the location of the distal end of the tunnel.
The myotomy length is generally 7 to 13 cm; however, it can be tailored for each patient. In type III achalasia, the myotomy length is longer to cover the entire spastic segment of the esophageal body. The most frequently used myotomy technique is partial myotomy, wherein only the circular muscle layer is dissected while preserving the longitudinal muscle layer.45 However, the longitudinal muscle fibers are often unintentionally damaged, resulting in gas leakage. Following the procedure, the incision site is closed using multiple hemostatic clips.
Minimal gas leakage is inevitable during the procedure because the esophagus does not have a serosal layer, and positive-pressure ventilation and CO2 insufflation are mandatory to minimize leakage. A multicenter retrospective study by Lee et al.46 reported gas-related minor complications in 22.8% of patients after POEM, with pneumoperitoneum (15.5%), the most common gas-related adverse effect, followed by subcutaneous emphysema (4.9%), pneumomediastinum (1.8%), and pneumoretroperitoneum (0.6%). However, minimal gas leaks generally do not affect the overall perioperative clinical course and tend to be absorbed naturally. The incidence of major complications is rare, at approximately 2.4%, and includes mucosal injury, bleeding, and hemothorax.46,47 Gastroesophageal reflux disease (GERD) is one of the major late complications of POEM, with a high prevalence rate of 29.4% to 39.7% (Fig. 6).48,49 Patients with symptomatic reflux are generally well-treated with proton pump inhibitors. Recently, concomitant endoscopic fundoplication following POEM was introduced, which showed a significantly lower incidence of GERD compared to conventional POEM after a 12-month follow-up.50,51
Accumulating data on POEM have demonstrated good treatment outcomes for short- and long-term symptom relief. A summary of previous studies comparing the long-term clinical efficacy of POEM is presented in Table 1.52-56 A large multicenter retrospective study by Shiwaku et al.57 analyzed the clinical outcomes of 1,346 patients who underwent POEM in Japan, including 18% with sigmoid-type achalasia and 31% with previous treatment histories. The study reported excellent response rates (Eckardt scores Ōēż3) of 95.1% at 3 months postoperatively and 94.7% at 1 year postoperatively.
Several studies have compared the outcomes of major treatment modalities. Ponds et al.58 conducted a multicenter prospective study in six different countries, in which 133 patients with achalasia were randomly assigned to either POEM or PD groups. The treatment response after the 2-year follow-up was significantly higher in the POEM group (92%) compared to that in the PD group (54%). A recent meta-analysis by Schlottmann et al.59 analyzed the response rates of 7,782 patients in 24 studies who underwent either laparoscopic Heller myotomy or POEM. Symptom improvement in dysphagia was observed in 93.5% of the patients who underwent POEM and 91.0% of those who underwent Heller myotomy at the 12-month follow-up and in 92.7% and 90.0% at the 24-month follow-up, respectively. However, POEM was associated with a significantly higher incidence of pathological reflux, both symptomatic reflux and GERD, as evidenced by pH monitoring. A multicenter comparative study by Kumbhari et al.60 compared the treatment outcomes of POEM with those of HellerŌĆÖs myotomy in patients with type III achalasia, in which POEM showed better clinical outcomes than HellerŌĆÖs myotomy, with response rates of 98.0% and 80.8%, respectively.
ENDOSCOPIC SURVEILLANCE
Despite the well-documented risk of esophageal cancer in patients with achalasia, the most recent American Society of Gastrointestinal Endoscopy guidelines concluded that routine endoscopic surveillance is insufficient because the overall absolute number of cancer incidences is low, as more than 400 endoscopies are required to detect one cancer.1,3,61
Many studies have suggested an association between esophageal squamous cell carcinoma (SCC) and achalasia, with 16- to 28-fold increased risks of esophageal carcinoma.62,63 Although the exact etiology remains unknown, the proposed mechanism is food stasis in the esophagus, which causes chronic inflammation and epithelial hyperplasia, eventually leading to SCC. The relationship between esophageal adenocarcinoma (EA) and achalasia is not yet well established; however, several studies have reported a substantial increase in the risk of EA, probably due to GERD after achalasia treatment, leading to the development of BarrettŌĆÖs esophagitis and, ultimately, EA.64,65
As concordant guidelines for surveillance are lacking, the duration and interval of surveillance are largely determined by physiciansŌĆÖ judgment. Experts generally recommend 3-yearly surveillance, but data on proper surveillance intervals are insufficient. In 2010, Leeuwenburgh et al.62 conducted a large cohort study of 448 patients with achalasia with a mean follow-up period of 9.6 years. Among these, 15 patients (3.3%) developed esophageal cancer (3 EA and 12 SCC) during follow-up after a mean of 11 years after the initial presentation. As six of the ten patients under the 3-yearly surveillance in the study died of esophageal cancer within 2 years, Leeuwenburgh et al.62 suggested the need for annual endoscopic surveillance of high-risk patients with achalasia starting 10 years after symptom onset.
To improve the accuracy of cancer detection in patients with achalasia, various modalities, including Lugol chromoendoscopy and narrow-band imaging (NBI), have been used for endoscopic surveillance. However, previous studies reported inconsistent outcomes regarding the effectiveness of cancer surveillance. Ide et al.66 showed equivalent performance in detecting esophageal SCC with NBI and LugolŌĆÖs staining, suggesting a potential role of NBI in detecting esophageal cancer in patients with achalasia. Another cohort study by Ponds et al.67 screened 230 patients with longstanding achalasia for esophageal dysplasia using both Lugol chromoendoscopy and conventional white-light endoscopy. Although Lugol chromoendoscopy showed a greater detection rate of suspected lesions (329 lesions vs. 111 lesions with white light), pathological analysis could only confirm 8% of them as true lesions.
Although surveillance for the sole purpose of cancer prevention or long-term survival benefits may not be cost-effective, many experts agree on the necessity of endoscopic surveillance for posttreatment symptom assessment, reflux evaluation, megaesophagus detection, and esophageal cancer screening.61 Further studies on the long-term benefits and methodologies of endoscopic surveillance are warranted.
CONCLUSIONS
The prevalence of achalasia is increasing worldwide; thus, the importance of accurate diagnosis, effective treatment, and careful follow-up of disease progression is growing. This study focused on the importance of endoscopy in achieving these goals.
Endoscopy is useful in the initial diagnosis to exclude other diseases that may cause symptoms similar to achalasia. The characteristic features of achalasia include luminal dilation, food impaction, and other recently introduced endoscopic findings such as pinstripe pattern and rosette-like esophageal folds. Once achalasia is diagnosed, patients can choose between endoscopic and surgical treatment. POEM is the most preferred treatment option among other methods due to its outstanding clinical outcomes and acceptable safety. However, the high prevalence of posttreatment GERD is a major drawback of POEM, and many studies have focused on modifying POEM to reduce its incidence. Concomitant endoscopic fundoplication following POEM, introduced by Inoue et al.,40 showed promising results in terms of both treatment success rate and low incidence of posttreatment GERD. Although remains no established consensus regarding the surveillance of patients with achalasia, there is a growing consensus regarding regular endoscopic surveillance for cancer screening and posttreatment symptom assessment.